Azo compound: Difference between revisions
CSV import |
CSV import |
||
| Line 28: | Line 28: | ||
[[Category:Pigments]] | [[Category:Pigments]] | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:Azo-group-2D-flat.png|Azo compound | |||
File:Phenazopyridine.svg|Phenazopyridine | |||
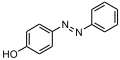
File:4-(Phenylazo)phenol structure.svg|4-(Phenylazo)phenol | |||
File:Formation of Radicals from AIBN.png|Formation of Radicals from AIBN | |||
</gallery> | |||
Latest revision as of 00:47, 20 February 2025
Azo compound is a type of chemical compound that contains a nitrogen double bond, represented as -N=N-. This functional group is the defining characteristic of azo compounds and is known as an azo group. Azo compounds are a significant class of industrial chemicals, particularly in dye manufacturing.
History[edit]
The first azo dye, aniline yellow, was produced in 1861 by Sir William Henry Perkin. This discovery marked the beginning of the synthetic dye industry, which has since developed a wide range of azo dyes and pigments.
Structure and Properties[edit]
Azo compounds are characterized by the presence of an azo group (-N=N-), which is a type of functional group in organic chemistry. The azo group is composed of two nitrogen atoms connected by a double bond. The azo group can link two organic groups, forming a stable compound.
The azo group is a strong chromophore, which means it absorbs light in the visible region of the electromagnetic spectrum. This property makes azo compounds useful in the production of dyes and pigments.
Synthesis[edit]
Azo compounds are typically synthesized through a process known as azo coupling. This reaction involves the reaction of an aromatic amine with a diazonium salt, resulting in the formation of an azo compound.
Applications[edit]
Azo compounds are widely used in the production of dyes and pigments. These compounds are responsible for the bright, vibrant colors found in many textiles, paints, and inks. Some azo compounds are also used in the food industry as food coloring agents.
Health and Environmental Concerns[edit]
Some azo compounds, particularly those used in the textile industry, have been associated with health and environmental concerns. Certain azo dyes can break down into aromatic amines, some of which are known to be carcinogenic. As a result, the use of these dyes is regulated in many countries.
-
Azo compound
-
Phenazopyridine
-
4-(Phenylazo)phenol
-
Formation of Radicals from AIBN