Decaborane: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import Tags: mobile edit mobile web edit |
||
| Line 25: | Line 25: | ||
[[Category:Inorganic compounds]] | [[Category:Inorganic compounds]] | ||
{{stub}} | {{stub}} | ||
<gallery> | |||
File:Decaborane(14)-from-xtal-view-1-tilt-3D-bs-17.png|Decaborane | |||
File:Decaborane.png|Decaborane | |||
</gallery> | |||
Latest revision as of 00:58, 18 February 2025
Decaborane, also known as Decaborane(14), is a chemical compound that belongs to the class of boranes. It is a colorless solid that is soluble in organic solvents. Decaborane is a cluster compound with unusual properties that make it useful in certain types of nuclear research and materials science.
Structure and Bonding[edit]
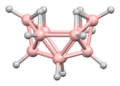
Decaborane(14) has a unique structure that is not shared by other boranes. It consists of a cluster of 10 boron atoms, arranged in a pattern that resembles a double pyramid. This structure is held together by 14 hydrogen atoms, which form bridges between the boron atoms. The boron-boron bonds within the cluster are multi-center bonds, which are a characteristic feature of boranes.
Synthesis[edit]
Decaborane can be synthesized from other boranes. One common method involves the reaction of diborane with a boron trihalide, such as boron trichloride or boron tribromide. The reaction is carried out at elevated temperatures and pressures, and the product is purified by sublimation.
Uses[edit]
Decaborane is used in a variety of applications. It is a precursor to other boron compounds, and it is also used in the production of boron-containing ceramics and glasses. In addition, decaborane is used as a reducing agent in organic synthesis, and as a dopant in the manufacture of semiconductors.
Safety[edit]
Decaborane is a highly reactive compound that can ignite spontaneously in air. It is also toxic if inhaled or ingested, and it can cause burns if it comes into contact with the skin or eyes. Therefore, it should be handled with care, and protective equipment should be worn when working with this compound.
See also[edit]
References[edit]
<references />