Nucleon: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 30: | Line 30: | ||
{{stub}} | {{stub}} | ||
{{dictionary-stub1}} | {{dictionary-stub1}} | ||
<gallery> | |||
File:Nucleus_drawing.svg|Nucleon | |||
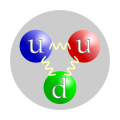
File:Quark_structure_proton.svg|Quark structure of a proton | |||
File:Quark_structure_neutron.svg|Quark structure of a neutron | |||
File:Quark_structure_antiproton.svg|Quark structure of an antiproton | |||
File:Quark_structure_antineutron.svg|Quark structure of an antineutron | |||
</gallery> | |||
Latest revision as of 04:38, 18 February 2025
Nucleon is a collective term for the particles that make up the nucleus of an atom, namely protons and neutrons. These particles are the most important components of atomic nuclei and are responsible for most of the mass of an atom.
Overview[edit]
Nucleons are composed of quarks, which are held together by the strong force. This force is one of the four fundamental forces of nature and is responsible for holding atomic nuclei together. Each nucleon is composed of three quarks: protons are composed of two up quarks and one down quark, while neutrons are composed of two down quarks and one up quark.
Properties[edit]
Nucleons have a number of important properties. They have a spin of 1/2, which means they are fermions. They also have a baryon number of 1, which distinguishes them from other particles such as mesons which have a baryon number of 0.
Nucleons also have a charge: protons have a charge of +1, while neutrons have a charge of 0. This charge is due to the charge of the quarks that make up the nucleon.
Interactions[edit]
Nucleons interact with each other through the strong force, which is mediated by particles called gluons. This force is responsible for holding the nucleons together in the nucleus of an atom.
Nucleons can also interact with other particles through the weak force, which is responsible for certain types of radioactive decay. For example, a neutron can decay into a proton, an electron, and an electron antineutrino through a process called beta decay.