Hemiacetal: Difference between revisions
CSV import |
CSV import |
||
| Line 20: | Line 20: | ||
{{stub}} | {{stub}} | ||
== Hemiacetal == | |||
<gallery> | |||
File:Hemiacetal_and_Hemiketal_structure.png|Hemiacetal and Hemiketal structure | |||
File:Formation_of_hemiacetals.png|Formation of hemiacetals | |||
File:Hemiketal_formation.png|Hemiketal formation | |||
File:Lactol_equilibrium.png|Lactol equilibrium | |||
File:RobustHemiacetals.svg|Robust Hemiacetals | |||
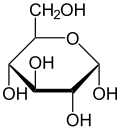
File:Alpha-D-Glucopyranose.svg|Alpha-D-Glucopyranose | |||
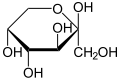
File:Beta-D-Fructopyranose.svg|Beta-D-Fructopyranose | |||
</gallery> | |||
Latest revision as of 21:21, 23 February 2025
Hemiacetal is a functional group in organic chemistry that consists of an alcohol and an ether connected to the same carbon atom. Hemiacetals are formed through the reaction of an aldehyde and an alcohol. They are unstable and often further react to form acetals.
Formation[edit]
Hemiacetals are formed through the reaction of an aldehyde or a ketone with an alcohol in the presence of acid. The reaction involves the addition of the alcohol to the carbonyl group, resulting in the formation of a tetrahedral intermediate. This intermediate then loses a proton to form the hemiacetal.
Properties[edit]
Hemiacetals are generally unstable and will further react to form acetals. However, in some cases, such as in the case of glucose, hemiacetals can be stable. This is due to the formation of a five or six-membered ring, which is energetically favorable.
Uses[edit]
Hemiacetals are important in the formation of many biological molecules. For example, glucose exists in solution primarily as a hemiacetal. They are also important in the formation of certain polymers, such as polyesters.