Beta sheet: Difference between revisions
CSV import |
CSV import |
||
| Line 25: | Line 25: | ||
{{stub}} | {{stub}} | ||
== Beta_sheet == | |||
<gallery> | |||
File:Animated_Beta_sheet.gif|Animated Beta sheet | |||
File:1gwe_antipar_betaSheet_both.png|1gwe antipar betaSheet both | |||
File:Ramachandran_plot_general_100K.jpg|Ramachandran plot general 100K | |||
File:Beta_sheet_bonding_antiparallel-color.svg|Beta sheet bonding antiparallel color | |||
File:Beta_sheet_bonding_parallel-color.svg|Beta sheet bonding parallel color | |||
File:Beta_hairpin.png|Beta hairpin | |||
File:Anthrax_toxin_protein_key_motif.svg|Anthrax toxin protein key motif | |||
File:beta-meander1.png|Beta meander 1 | |||
</gallery> | |||
Latest revision as of 00:52, 27 February 2025
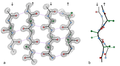
Beta sheet is a common motif of regular secondary structure in proteins. Beta sheets consist of beta strands (also β-strand) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in formation of the protein aggregates and fibrils observed in many human diseases, notably the amyloidoses such as Alzheimer's disease.
Structure[edit]
Beta sheets are formed by beta strands connected laterally by two or three backbone hydrogen bonds. The strands may be parallel (running in the same direction) or anti-parallel (running in opposite directions). The pleating of the sheet is due to the alternating directions of the side chains of the amino acids.
Stability[edit]
The stability of the beta sheet structure is determined by the hydrogen bonds that form between the carbonyl oxygen of one amino acid and the amino hydrogen of another. These bonds are almost perpendicular to the plane of the sheet, contributing to the pleated appearance.
Function[edit]
Beta sheets are present in all classes of proteins, with a particular function depending on the specific protein. They provide structural support and can also participate in protein-protein interactions.
Diseases[edit]
The supramolecular association of β-sheets has been implicated in formation of the protein aggregates and fibrils observed in many human diseases, notably the amyloidoses such as Alzheimer's disease.