Alpha 2-antiplasmin: Difference between revisions
CSV import |
CSV import |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''Alpha-2-antiplasmin''' is a [[serine protease inhibitor]] (serpin) that plays a crucial role in the regulation of the [[fibrinolytic system]]. It is primarily responsible for inhibiting [[plasmin]], the enzyme that degrades [[fibrin]] clots. By controlling plasmin activity, alpha-2-antiplasmin helps maintain the balance between clot formation and clot dissolution, which is essential for normal [[hemostasis]]. | '''Alpha-2-antiplasmin''' is a [[serine protease inhibitor]] (serpin) that plays a crucial role in the regulation of the [[fibrinolytic system]]. It is primarily responsible for inhibiting [[plasmin]], the enzyme that degrades [[fibrin]] clots. By controlling plasmin activity, alpha-2-antiplasmin helps maintain the balance between clot formation and clot dissolution, which is essential for normal [[hemostasis]]. | ||
| Line 14: | Line 10: | ||
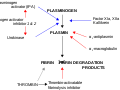
Fibrinolysis is the process by which fibrin clots are broken down in the body. This process is essential for wound healing and the prevention of thrombosis. Alpha-2-antiplasmin is a major inhibitor of fibrinolysis, acting by binding to plasmin and preventing it from degrading fibrin. | Fibrinolysis is the process by which fibrin clots are broken down in the body. This process is essential for wound healing and the prevention of thrombosis. Alpha-2-antiplasmin is a major inhibitor of fibrinolysis, acting by binding to plasmin and preventing it from degrading fibrin. | ||
In the fibrinolytic pathway, plasminogen is activated to plasmin by tissue plasminogen activator (tPA) or urokinase. Plasmin then degrades fibrin into soluble fragments. Alpha-2-antiplasmin rapidly inhibits free plasmin, ensuring that fibrinolysis is localized to the site of the clot and does not lead to systemic bleeding. | In the fibrinolytic pathway, plasminogen is activated to plasmin by tissue plasminogen activator (tPA) or urokinase. Plasmin then degrades fibrin into soluble fragments. Alpha-2-antiplasmin rapidly inhibits free plasmin, ensuring that fibrinolysis is localized to the site of the clot and does not lead to systemic bleeding. | ||
| Line 35: | Line 29: | ||
[[Category:Proteins]] | [[Category:Proteins]] | ||
[[Category:Hematology]] | [[Category:Hematology]] | ||
== Alpha_2-antiplasmin == | |||
<gallery> | |||
File:Fibrinolysis.svg|Fibrinolysis | |||
</gallery> | |||
Latest revision as of 20:56, 25 February 2025
Alpha-2-antiplasmin is a serine protease inhibitor (serpin) that plays a crucial role in the regulation of the fibrinolytic system. It is primarily responsible for inhibiting plasmin, the enzyme that degrades fibrin clots. By controlling plasmin activity, alpha-2-antiplasmin helps maintain the balance between clot formation and clot dissolution, which is essential for normal hemostasis.
Structure and Function[edit]
Alpha-2-antiplasmin is a glycoprotein synthesized mainly in the liver. It circulates in the blood plasma and is composed of a single polypeptide chain. The primary function of alpha-2-antiplasmin is to inhibit plasmin, thereby preventing excessive fibrinolysis and ensuring that blood clots are stable enough to prevent bleeding.
The interaction between alpha-2-antiplasmin and plasmin is a key component of the fibrinolytic system. When a blood clot forms, plasminogen is converted to plasmin, which then degrades fibrin. Alpha-2-antiplasmin binds to plasmin, inhibiting its activity and thus regulating the breakdown of fibrin clots.
Role in Fibrinolysis[edit]
Fibrinolysis is the process by which fibrin clots are broken down in the body. This process is essential for wound healing and the prevention of thrombosis. Alpha-2-antiplasmin is a major inhibitor of fibrinolysis, acting by binding to plasmin and preventing it from degrading fibrin.
In the fibrinolytic pathway, plasminogen is activated to plasmin by tissue plasminogen activator (tPA) or urokinase. Plasmin then degrades fibrin into soluble fragments. Alpha-2-antiplasmin rapidly inhibits free plasmin, ensuring that fibrinolysis is localized to the site of the clot and does not lead to systemic bleeding.
Clinical Significance[edit]
Deficiencies or dysfunctions in alpha-2-antiplasmin can lead to bleeding disorders due to excessive fibrinolysis. Conversely, elevated levels of alpha-2-antiplasmin can contribute to thrombotic conditions by preventing the normal breakdown of clots.
Alpha-2-antiplasmin levels can be measured in the laboratory to assess fibrinolytic activity and diagnose bleeding disorders. Therapeutic modulation of alpha-2-antiplasmin activity is a potential strategy for managing conditions such as deep vein thrombosis and pulmonary embolism.
Related Pages[edit]
Alpha_2-antiplasmin[edit]
-
Fibrinolysis