Osmium hexafluoride: Difference between revisions
CSV import |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 28: | Line 28: | ||
{{chemistry-stub}} | {{chemistry-stub}} | ||
<gallery> | |||
File:Osmium(VI)-fluoride.svg|Osmium(VI)-fluoride.svg | |||
File:Osmium(VI)-fluorid.png|Osmium(VI)-fluorid.png | |||
</gallery> | |||
Latest revision as of 00:48, 17 March 2025
Osmium hexafluoride (OsF6) is a chemical compound of osmium and fluorine. It is one of the few binary hexafluorides, a class of compounds where six fluorine atoms are bonded to a central metal atom. Osmium hexafluoride is notable for its role in the study of chemical bonding and molecular geometry, as well as its applications in various chemical processes.
Properties[edit]
Osmium hexafluoride is a volatile, crystalline solid at room temperature. It has a high melting point and sublimes at temperatures slightly above room temperature, making it a challenging substance to work with. The compound is highly reactive, especially with water, releasing toxic gases. It is one of the most potent oxidizing agents known and must be handled with extreme care in a controlled environment.
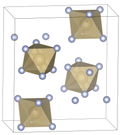
The molecular geometry of OsF6 is octahedral, a common shape for compounds with six ligands surrounding a central atom. This geometry is significant in the field of coordination chemistry and provides insights into the electronic structure of the osmium atom when it is in a high oxidation state.
Synthesis[edit]
Osmium hexafluoride is synthesized through the direct reaction of osmium metal with fluorine gas. This process requires careful control of reaction conditions, including temperature and pressure, to ensure the safety and yield of the product. The reaction is highly exothermic, releasing a significant amount of energy.
Applications[edit]
While osmium hexafluoride itself has limited applications due to its reactivity and toxicity, its study has contributed to the broader understanding of chemical bonding, molecular geometry, and the behavior of hexafluorides in general. Its properties are of interest in the development of new materials and in the field of inorganic chemistry.
Safety[edit]
Due to its high reactivity and toxicity, osmium hexafluoride requires stringent safety measures during handling and storage. It can cause severe burns upon contact with skin and is dangerous if inhaled. Laboratories working with OsF6 must be equipped with appropriate safety equipment, including fume hoods and protective clothing.
See also[edit]
-
Osmium(VI)-fluoride.svg
-
Osmium(VI)-fluorid.png