FUB-144: Difference between revisions
CSV import |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 25: | Line 25: | ||
{{stub}} | {{stub}} | ||
<gallery> | |||
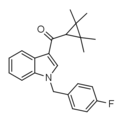
File:FUB-144 structure.png|FUB-144 Structure | |||
</gallery> | |||
Latest revision as of 21:02, 16 March 2025
FUB-144 is a type of indole-based synthetic cannabinoid, which is believed to act as a potent agonist for the CB1 receptor. It was originally developed by Pfizer in 2009 as an analgesic medication, but has since been found in synthetic cannabis products.
History[edit]
FUB-144 was first synthesized and patented by Pfizer in 2009. It was developed as part of a wider range of analgesic medications, but was not pursued for human use. In 2012, FUB-144 started appearing in synthetic cannabis products in Japan.
Pharmacology[edit]
FUB-144 is an indole-based synthetic cannabinoid that is presumed to be a potent agonist of the CB1 receptor. Because of its structure, it is believed to bind strongly with the CB1 receptor, giving it its psychoactive effects. However, the exact mechanism of action is not fully understood.
Legal Status[edit]
FUB-144 is illegal in many countries, including the United States, where it is a Schedule I controlled substance. It is also banned in many European countries, as well as in Japan, where it first appeared in synthetic cannabis products.
Health Risks[edit]
The health risks associated with FUB-144 are not well-studied. However, synthetic cannabinoids in general are known to be dangerous and unpredictable, with potential side effects including seizures, psychosis, and even death.