HIV-1 protease: Difference between revisions
CSV import |
CSV import |
||
| Line 23: | Line 23: | ||
[[Category:Virology]] | [[Category:Virology]] | ||
{{pharma-stub}} | {{pharma-stub}} | ||
== HIV-1_protease == | |||
<gallery> | |||
File:Aspartic_protease.png|Aspartic protease | |||
File:molecular_bulldog_face.png|Molecular bulldog face | |||
File:Hxb2genome.gif|Hxb2 genome | |||
File:HIV_protease_1KJF.png|HIV protease 1KJF | |||
File:HIV_protease_1EBY.png|HIV protease 1EBY | |||
File:Aspartyl_protease_mechanism.png|Aspartyl protease mechanism | |||
File:HIV-1_Protease_with_Active_Site.png|HIV-1 Protease with Active Site | |||
</gallery> | |||
Latest revision as of 20:55, 25 February 2025
HIV-1 protease is an enzyme that plays a critical role in the life cycle of the Human Immunodeficiency Virus Type 1 (HIV-1), which is the virus responsible for the Acquired Immunodeficiency Syndrome (AIDS). This enzyme is essential for the post-translational processing of the viral polyproteins, leading to the maturation of the virus and its ability to infect new cells. Due to its crucial role in the viral life cycle, HIV-1 protease has been a prime target for antiretroviral therapy aimed at treating HIV/AIDS.
Function[edit]
HIV-1 protease is a dimer, consisting of two identical subunits, each comprising 99 amino acids. It cleaves the Gag and Gag-Pol polyproteins at specific sites, leading to the structural proteins and enzymes necessary for the assembly of new virions. Without the activity of HIV-1 protease, the viral particles remain immature and non-infectious.
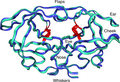
Structure[edit]
The enzyme's active site is located in a cleft between the two subunits, and it has a unique Aspartyl protease mechanism, utilizing two aspartate residues to cleave the peptide bond. The specificity of HIV-1 protease for its substrates is highly precise, making it an attractive target for drug design.
Inhibition and Drug Resistance[edit]
The inhibition of HIV-1 protease disrupts the viral replication cycle, making protease inhibitors (PIs) a key component of Highly Active Antiretroviral Therapy (HAART). However, the high mutation rate of HIV-1 leads to the emergence of drug-resistant strains, posing significant challenges to the effectiveness of PIs. Continuous efforts in drug development are necessary to overcome resistance and improve treatment outcomes.
Clinical Significance[edit]
The discovery of HIV-1 protease and its role in the viral life cycle has had a profound impact on the management of HIV/AIDS. Protease inhibitors have significantly improved the life expectancy and quality of life for individuals living with HIV. Ongoing research focuses on developing new inhibitors with better efficacy and resistance profiles, as well as understanding the mechanisms of drug resistance.
See Also[edit]
HIV-1_protease[edit]
-
Aspartic protease
-
Molecular bulldog face
-
Hxb2 genome
-
HIV protease 1KJF
-
HIV protease 1EBY
-
Aspartyl protease mechanism
-
HIV-1 Protease with Active Site