JUNQ and IPOD: Difference between revisions
CSV import |
CSV import |
||
| Line 20: | Line 20: | ||
{{Molecular-biology-stub}} | {{Molecular-biology-stub}} | ||
<gallery> | |||
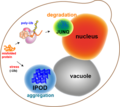
File:A_scheme_of_a_yeast_cell_harboring_JUNQ_and_IPOD_inclusions.png|A scheme of a yeast cell harboring JUNQ and IPOD inclusions | |||
File:A_cell_harboring_JUNQ_and_IPOD_inclusions.png|A cell harboring JUNQ and IPOD inclusions | |||
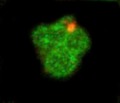
File:JUNQ_(green)_tethered_to_the_nucleus_(orange).tif|JUNQ (green) tethered to the nucleus (orange) | |||
File:IPOD_(red)_tethered_to_the_vacuole_(green).tif|IPOD (red) tethered to the vacuole (green) | |||
</gallery> | |||
Latest revision as of 05:02, 18 February 2025
JUNQ and IPOD are cellular structures involved in the management of misfolded proteins, which are crucial for maintaining cellular health and preventing diseases. Misfolded proteins can arise from various stressors or genetic mutations and are implicated in numerous diseases, including neurodegenerative disorders like Alzheimer's and Parkinson's disease.
JUNQ[edit]
The Juxtanuclear Quality Control Compartment (JUNQ) is a cellular structure that isolates misfolded proteins that are still potentially salvageable. These proteins are targeted for refolding by molecular chaperones. The JUNQ compartment is located near the nucleus, facilitating the distinction between proteins that can be salvaged and those that are irreversibly damaged and need to be degraded. Proteins that fail to refold correctly are then transferred to the proteasome for degradation.
IPOD[edit]
The Insoluble Protein Deposit (IPOD) is another cellular structure, distinct from the JUNQ, where terminally misfolded proteins are sequestered. These proteins are typically rich in beta-sheet structures and are prone to forming amyloid-like aggregates. The IPOD is thought to play a protective role by sequestering these potentially toxic proteins, thereby preventing them from forming harmful aggregates within the cell. Proteins localized to the IPOD are often beyond repair and are not targeted for refolding or degradation.
Function and Importance[edit]
The distinction between JUNQ and IPOD is crucial for cellular homeostasis and the prevention of protein aggregation diseases. By segregating misfolded proteins based on their potential for refolding and degradation, cells can efficiently manage protein quality control and reduce the burden of protein aggregation. This segregation also helps in understanding the mechanisms underlying various neurodegenerative diseases and in developing therapeutic strategies targeting the pathways involved in protein misfolding and aggregation.
Relation to Diseases[edit]
Misfolded proteins and their aggregation are central to the pathology of several neurodegenerative diseases. The dysfunction of JUNQ and IPOD compartments can lead to the accumulation of toxic protein aggregates, contributing to cell death and disease progression. Research into these compartments offers insights into the cellular mechanisms that fail in disease states, providing potential targets for therapeutic intervention.
Research and Therapeutic Implications[edit]
Understanding the mechanisms governing the function of JUNQ and IPOD and their role in protein quality control has significant implications for developing treatments for diseases associated with protein misfolding and aggregation. Enhancing the capacity of these compartments to manage misfolded proteins or preventing the misfolding in the first place could be potential strategies for therapeutic intervention.

This article is a molecular biology stub. You can help WikiMD by expanding it!
-
A scheme of a yeast cell harboring JUNQ and IPOD inclusions
-
A cell harboring JUNQ and IPOD inclusions
-
JUNQ (green) tethered to the nucleus (orange)
-
IPOD (red) tethered to the vacuole (green)