Uronic acid: Difference between revisions
CSV import |
CSV import |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Uronic Acid == | |||
[[File:Beta_D-Glucuronic_acid.svg|thumb|right|200px|Structure of Beta-D-Glucuronic acid]] | |||
[[File:Beta-D-Glucose.svg|thumb|right|200px|Structure of Beta-D-Glucose]] | |||
'''Uronic acids''' are a class of [[sugar acids]] with both a carbonyl and a carboxylic acid functional group. They are derived from [[monosaccharides]] by oxidation of the terminal hydroxyl group to a carboxylic acid. Uronic acids are important in the metabolism of [[carbohydrates]] and are found in many [[polysaccharides]]. | |||
== | == Structure and Properties == | ||
Uronic acids are characterized by the presence of a carboxylic acid group at the terminal carbon of the sugar molecule. This modification increases the molecule's solubility in water and alters its chemical reactivity. The most common uronic acids are derived from [[hexoses]], such as [[glucose]] and [[galactose]]. | |||
The | |||
== | == Biological Role == | ||
== | Uronic acids play a crucial role in the [[metabolism]] of carbohydrates. They are involved in the detoxification of substances in the [[liver]] through the formation of [[glucuronides]], which are more water-soluble and can be excreted in the [[urine]]. | ||
* [[ | |||
== Examples == | |||
* '''[[Glucuronic acid]]''' is a prominent uronic acid derived from glucose. It is a key component of [[glycosaminoglycans]], such as [[hyaluronic acid]] and [[chondroitin sulfate]], which are important for the structure and function of [[connective tissues]]. | |||
* '''[[Galacturonic acid]]''' is derived from galactose and is a major component of [[pectin]], a polysaccharide found in the cell walls of plants. | |||
== Related Compounds == | |||
Uronic acids are related to other sugar acids, such as [[aldonic acids]] and [[aldaric acids]], which are formed by oxidation of different carbon atoms in the sugar molecule. | |||
== Related Pages == | |||
* [[Carbohydrate metabolism]] | |||
* [[Glucuronidation]] | |||
* [[Polysaccharide]] | * [[Polysaccharide]] | ||
== References == | |||
{{Reflist}} | |||
[[Category:Carbohydrates]] | [[Category:Carbohydrates]] | ||
[[Category:Sugar acids]] | |||
<gallery> | |||
File:Beta_D-Glucuronic_acid.svg|Beta-D-Glucuronic acid | |||
File:Beta-D-Glucose.svg|Beta-D-Glucose | |||
</gallery> | |||
Latest revision as of 02:01, 17 February 2025
Uronic Acid[edit]

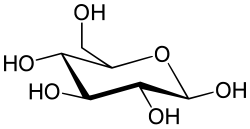
Uronic acids are a class of sugar acids with both a carbonyl and a carboxylic acid functional group. They are derived from monosaccharides by oxidation of the terminal hydroxyl group to a carboxylic acid. Uronic acids are important in the metabolism of carbohydrates and are found in many polysaccharides.
Structure and Properties[edit]
Uronic acids are characterized by the presence of a carboxylic acid group at the terminal carbon of the sugar molecule. This modification increases the molecule's solubility in water and alters its chemical reactivity. The most common uronic acids are derived from hexoses, such as glucose and galactose.
Biological Role[edit]
Uronic acids play a crucial role in the metabolism of carbohydrates. They are involved in the detoxification of substances in the liver through the formation of glucuronides, which are more water-soluble and can be excreted in the urine.
Examples[edit]
- Glucuronic acid is a prominent uronic acid derived from glucose. It is a key component of glycosaminoglycans, such as hyaluronic acid and chondroitin sulfate, which are important for the structure and function of connective tissues.
- Galacturonic acid is derived from galactose and is a major component of pectin, a polysaccharide found in the cell walls of plants.
Related Compounds[edit]
Uronic acids are related to other sugar acids, such as aldonic acids and aldaric acids, which are formed by oxidation of different carbon atoms in the sugar molecule.
Related Pages[edit]
References[edit]
<references group="" responsive="1"></references>
-
Beta-D-Glucuronic acid
-
Beta-D-Glucose

