Ring expansion and contraction: Difference between revisions
CSV import |
CSV import |
||
| Line 45: | Line 45: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:Key step of pinacol mechanism.jpg|Key step of pinacol mechanism | |||
File:Ring expansion mechanisms.jpg|Ring expansion mechanisms | |||
File:General Buchner ring expansion.jpg|General Buchner ring expansion | |||
File:Beckmann-rearangement (cropped).png|Beckmann rearrangement | |||
File:Nitrosodecarboxylation Caprolactam Synthesis.svg|Nitrosodecarboxylation Caprolactam Synthesis | |||
File:Schmidt ring expansion.png|Schmidt ring expansion | |||
File:RIng contraction mechanisms.jpg|Ring contraction mechanisms | |||
File:Generalized Wolf rearrangement mechanism.jpg|Generalized Wolf rearrangement mechanism | |||
File:Generalized Favorskii rearrangement mechansim.jpg|Generalized Favorskii rearrangement mechanism | |||
File:Pinacol Rearrangment to a 5,7 ring system.jpg|Pinacol Rearrangement to a 5,7 ring system | |||
</gallery> | |||
Latest revision as of 05:38, 3 March 2025
Ring expansion and ring contraction are chemical reactions that alter the size of a cyclic molecule by increasing or decreasing the number of atoms in the ring. These reactions are significant in organic chemistry, particularly in the synthesis of cyclic compounds, which are common in both natural products and synthetic pharmaceuticals. Understanding these processes is crucial for designing synthetic routes to complex molecules.
Mechanisms of Ring Expansion[edit]
Ring expansion typically occurs through a series of steps involving the generation of a more stable carbocation intermediate. The most common mechanisms include:
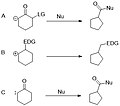
- Wagner-Meerwein rearrangement: This involves the migration of an alkyl or aryl group from one carbon to a neighboring carbon, resulting in the expansion of the ring. The driving force is the formation of a more stable carbocation intermediate.
- Pinacol rearrangement: In this reaction, a 1,2-diol is converted into a ketone, facilitating the expansion of the ring. This rearrangement involves the migration of a group adjacent to a carbocation generated by the loss of a leaving group.
- Beckmann rearrangement: Although primarily known for converting ketoximes to amides, this rearrangement can also lead to ring expansion when applied to cyclic ketoximes.
Mechanisms of Ring Contraction[edit]
Ring contraction reactions are less common than ring expansions but are equally important. They typically involve:
- Favorskii rearrangement: This reaction involves the contraction of a ring through the action of a base on a haloketone, forming a smaller ring and an alkene.
- Schmidt reaction: This reaction involves the reaction of hydrazoic acid with ketones or aldehydes to form smaller rings and nitrogen gas.
- Ring contraction via carbocation rearrangement: Similar to ring expansion, ring contraction can also occur through carbocation intermediates, where the ring contracts to form a more stable carbocation.
Applications in Synthesis[edit]
Ring expansion and contraction reactions are powerful tools in the synthesis of complex organic molecules. They allow chemists to:
- Access cyclic compounds of different sizes, which are crucial in the synthesis of natural products and pharmaceuticals.
- Modify the ring size to influence the biological activity and physical properties of a compound.
- Generate structural diversity in synthetic libraries for drug discovery.
Challenges and Considerations[edit]
While ring expansion and contraction provide versatile methods for modifying cyclic structures, they also present challenges:
- Regioselectivity and stereoselectivity can be difficult to control in these reactions.
- The formation of undesired by-products or isomers may complicate purification.
- The stability of intermediates and the potential for rearrangement reactions must be carefully considered.
Conclusion[edit]
Ring expansion and contraction are essential reactions in organic chemistry, offering pathways to synthesize cyclic compounds of varying sizes. These reactions are instrumental in the development of new drugs and materials, highlighting the importance of understanding their mechanisms and applications.
-
Key step of pinacol mechanism
-
Ring expansion mechanisms
-
General Buchner ring expansion
-
Beckmann rearrangement
-
Nitrosodecarboxylation Caprolactam Synthesis
-
Schmidt ring expansion
-
Ring contraction mechanisms
-
Generalized Wolf rearrangement mechanism
-
Generalized Favorskii rearrangement mechanism
-
Pinacol Rearrangement to a 5,7 ring system