Dess–Martin oxidation: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 30: | Line 30: | ||
[[Category:Oxidation reactions]] | [[Category:Oxidation reactions]] | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:Reaction_scheme.png|Reaction scheme for Dess–Martin oxidation | |||
File:Dess-Martin_oxidation.svg|Diagram of Dess–Martin oxidation | |||
File:Asthma_Inhaler_(29172634251).jpg|Image of an asthma inhaler | |||
</gallery> | |||
Latest revision as of 00:52, 18 February 2025
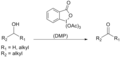
Dess–Martin Oxidation is a chemical reaction that involves the oxidation of primary and secondary alcohols to aldehydes and ketones, respectively. This reaction is highly selective, efficient, and proceeds under mild conditions, making it a valuable tool in organic synthesis, particularly in the synthesis of fine chemicals and pharmaceuticals. The Dess–Martin Periodinane (DMP), the reagent used in this oxidation, is a stable and powerful oxidizing agent that has found widespread application in organic chemistry since its introduction.
Overview[edit]
The Dess–Martin Oxidation was first reported in 1983 by Daniel Benjamin Dess and James Cullen Martin. The reaction utilizes Dess–Martin Periodinane (DMP), an iodine(V) reagent, for the efficient oxidation of alcohols to the corresponding carbonyl compounds. The reaction mechanism involves the formation of an alkoxyl–iodine(III) intermediate, which then undergoes a rearrangement to produce the carbonyl product and release iodobenzene and acetic acid as byproducts.
Reaction Mechanism[edit]
The mechanism of the Dess–Martin Oxidation begins with the alcohol oxygen attacking the iodine center of DMP, leading to the formation of an alkoxyl–iodine(III) species. This intermediate then undergoes a two-electron oxidation process, where the bond between the oxygen and the carbon of the alcohol is cleaved, forming the carbonyl compound. The byproducts of this reaction are iodobenzene and acetic acid, which are typically easy to separate from the desired product.
Applications[edit]
Dess–Martin Oxidation has found extensive use in organic synthesis due to its mild reaction conditions and high selectivity. It is particularly useful in the synthesis of sensitive molecules where traditional oxidation methods may cause degradation or unwanted side reactions. The reaction has been employed in the synthesis of complex natural products, pharmaceuticals, and other fine chemicals.
Advantages[edit]
- Selectivity: The reaction selectively oxidizes primary and secondary alcohols to aldehydes and ketones, respectively, without overoxidation to carboxylic acids.
- Mild Conditions: The reaction proceeds under mild conditions, minimizing the risk of damaging sensitive functional groups.
- High Efficiency: Dess–Martin Oxidation typically yields high conversions and selectivities, making it an efficient method for obtaining carbonyl compounds.
Limitations[edit]
While the Dess–Martin Oxidation is a powerful tool, it is not without limitations. The cost of the DMP reagent can be a consideration for large-scale applications. Additionally, the reaction conditions may need to be carefully optimized for substrates with sensitive or reactive functional groups to avoid side reactions.
See Also[edit]
References[edit]
<references/>
-
Reaction scheme for Dess–Martin oxidation
-
Diagram of Dess–Martin oxidation
-
Image of an asthma inhaler