Lead dioxide: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 31: | Line 31: | ||
{{Chem-stub}} | {{Chem-stub}} | ||
<gallery> | |||
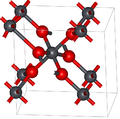
File:ScrutinyiteStructure.png|Scrutinyite Structure | |||
File:PlattneriteStructure.png|Plattnerite Structure | |||
</gallery> | |||
Latest revision as of 01:58, 17 February 2025
Lead dioxide (PbO2), also known as plumbic oxide, is a chemical compound that is a dark-brown solid which is insoluble in water. It exists in two crystalline forms. The alpha phase has an orthorhombic crystal structure, and the beta phase has a tetragonal structure. Lead dioxide is an oxidizing agent that is used in the production of lead acid batteries and various other applications, including the manufacture of matches and fireworks, and in the treatment of certain types of industrial waste.
Properties[edit]
Lead dioxide is a strong oxidizer, which enables it to react with acids releasing oxygen. In addition to its use in the production of lead acid batteries, where it serves as the positive plate material, PbO2 is also utilized in certain types of electrochemical cells and as a catalyst in organic synthesis.
Production[edit]
Lead dioxide can be produced by several methods. The most common method involves the oxidation of lead(II) oxide (PbO) with chlorine or by electrolysis of lead(II) nitrate solution. Another method is the thermal decomposition of lead(II) nitrate, which yields lead(II) oxide that can be further oxidized to lead dioxide.
Applications[edit]
Lead Acid Batteries[edit]
The primary use of lead dioxide is in the manufacture of lead acid batteries. In these batteries, PbO2 serves as the material for the positive electrode, while the negative electrode is made of metallic lead. When the battery is discharged, both electrodes are converted to lead(II) sulfate (PbSO4).
Chemical Synthesis and Treatment[edit]
Lead dioxide is used as a catalyst in the synthesis of certain organic compounds. It is also employed in the treatment of industrial waste, where its strong oxidative properties allow for the breakdown of organic pollutants.
Health and Environmental Concerns[edit]
Lead dioxide, like other lead compounds, is toxic and poses significant health and environmental risks. Inhalation or ingestion of lead dioxide can lead to lead poisoning, which affects the nervous system, blood production, and kidneys. Due to its toxicity, the use and disposal of lead dioxide must be carefully managed to prevent environmental contamination and human exposure.
Regulation[edit]
The use and disposal of lead dioxide are subject to regulation in many countries. Regulations may include limits on emissions, guidelines for safe handling and disposal, and requirements for protective equipment for workers handling the compound.
See Also[edit]
-
Scrutinyite Structure
-
Plattnerite Structure