Benzylidene compounds: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import Tags: mobile edit mobile web edit |
||
| Line 20: | Line 20: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
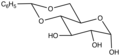
File:Bngluc.png|Benzylidene glucoside | |||
</gallery> | |||
Latest revision as of 06:21, 3 March 2025
Benzylidene compounds are a class of organic chemical compounds characterized by the presence of a benzylidene group, which is a form of alkene where one of the carbon atoms is part of a benzene ring and the other is part of a smaller alkyl chain. These compounds are significant in various fields of chemistry, including synthetic chemistry, medicinal chemistry, and material science. They are involved in numerous chemical reactions and are pivotal in the synthesis of a wide range of chemicals, including pharmaceuticals, fragrances, and advanced materials.
Structure and Nomenclature[edit]
The general structure of benzylidene compounds can be represented as C6H5CH=CHR, where C6H5 represents the phenyl group, and CHR represents an alkyl group or hydrogen. The double bond between the carbon atoms makes these compounds unsaturated. The nomenclature of benzylidene compounds follows the IUPAC rules, where the name typically includes the term "benzylidene" followed by the name of the alkyl group.
Synthesis[edit]
Benzylidene compounds are synthesized through various chemical reactions, the most common being the Aldol condensation. This reaction involves the condensation of an aldehyde or ketone with a compound containing an active hydrogen in the presence of a base. Another method is through the Wittig reaction, where a phosphonium ylide reacts with an aldehyde or ketone to form a benzylidene compound.
Applications[edit]
Benzylidene compounds have diverse applications due to their chemical properties. In medicinal chemistry, they are used in the synthesis of various pharmaceuticals due to their ability to interact with biological molecules. They also find applications in the fragrance industry, where they are used to synthesize compounds with desirable scents. Furthermore, in material science, benzylidene compounds are used in the creation of polymers and advanced materials with specific optical and electronic properties.
Safety and Environmental Impact[edit]
Like many chemical compounds, benzylidene compounds must be handled with care. They can be hazardous if inhaled, ingested, or come into contact with skin. Proper safety protocols, including the use of personal protective equipment (PPE), are essential when working with these compounds. Additionally, their environmental impact should be considered, as some benzylidene compounds may be persistent in the environment or toxic to aquatic life.
Conclusion[edit]
Benzylidene compounds play a crucial role in various branches of chemistry and have significant applications in the synthesis of pharmaceuticals, fragrances, and advanced materials. Their synthesis, properties, and applications make them an important topic of study in organic chemistry.
-
Benzylidene glucoside