Vancosamine: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''Vancosamine''' is a | == Vancosamine == | ||
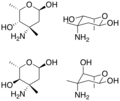
[[File:Vancosamine_and_epivancosamine.png|thumb|right|Vancosamine and epivancosamine structures]] | |||
'''Vancosamine''' is a sugar derivative that is part of the glycopeptide antibiotic [[vancomycin]]. It is a deoxy sugar, specifically a 3-amino-2,3,6-trideoxyhexose, and plays a crucial role in the activity of vancomycin by contributing to its ability to bind to bacterial cell walls. | |||
== Structure and Properties == | == Structure and Properties == | ||
Vancosamine is | Vancosamine is characterized by its unique structure, which includes an amino group at the C-3 position and the absence of hydroxyl groups at the C-2, C-3, and C-6 positions. This structure is essential for its function in antibiotics, as it allows for specific interactions with bacterial targets. | ||
== Biosynthesis == | |||
The structure of vancosamine | The biosynthesis of vancosamine involves several enzymatic steps that convert simple sugar precursors into the complex structure of vancosamine. The process begins with the formation of a nucleotide-activated sugar, which undergoes a series of modifications, including deoxygenation and amination. | ||
== | === Initial Steps === | ||
[[File:Vancosamine_biosynth_part_1.png|thumb|right|Initial steps in vancosamine biosynthesis]] | |||
The biosynthesis pathway starts with the conversion of a common sugar nucleotide, such as [[UDP-glucose]], into a deoxygenated form. This is achieved through the action of specific dehydratase enzymes that remove hydroxyl groups. | |||
== | === Amination and Final Modifications === | ||
[[File:Vancosamine_biosynthesis_part_2.png|thumb|right|Amination and final modifications in vancosamine biosynthesis]] | |||
== | Subsequent steps involve the introduction of an amino group, typically through the action of transaminase enzymes. The final structure of vancosamine is achieved after several more modifications, including the addition of methyl groups and further deoxygenation. | ||
== Role in Antibiotics == | |||
Vancosamine is a critical component of the glycopeptide antibiotic vancomycin. It enhances the antibiotic's ability to bind to the D-Ala-D-Ala terminus of bacterial cell wall precursors, thereby inhibiting cell wall synthesis and exerting its antibacterial effects. This makes vancomycin an important treatment option for infections caused by [[Gram-positive bacteria]], including [[methicillin-resistant Staphylococcus aureus]] (MRSA). | |||
== Related Pages == | |||
* [[Vancomycin]] | * [[Vancomycin]] | ||
* [[ | * [[Glycopeptide antibiotics]] | ||
* [[ | * [[Bacterial cell wall synthesis]] | ||
[[Category:Antibiotics]] | |||
[[Category:Carbohydrates]] | |||
<gallery> | |||
File:Vancosamine_and_epivancosamine.png|Vancosamine and Epivancosamine | |||
File:Vancosamine_biosynth_part_1.png|Vancosamine Biosynthesis Part 1 | |||
File:Vancosamine_biosynthesis_part_2.png|Vancosamine Biosynthesis Part 2 | |||
</gallery> | |||
Latest revision as of 04:02, 18 February 2025
Vancosamine[edit]

Vancosamine is a sugar derivative that is part of the glycopeptide antibiotic vancomycin. It is a deoxy sugar, specifically a 3-amino-2,3,6-trideoxyhexose, and plays a crucial role in the activity of vancomycin by contributing to its ability to bind to bacterial cell walls.
Structure and Properties[edit]
Vancosamine is characterized by its unique structure, which includes an amino group at the C-3 position and the absence of hydroxyl groups at the C-2, C-3, and C-6 positions. This structure is essential for its function in antibiotics, as it allows for specific interactions with bacterial targets.
Biosynthesis[edit]
The biosynthesis of vancosamine involves several enzymatic steps that convert simple sugar precursors into the complex structure of vancosamine. The process begins with the formation of a nucleotide-activated sugar, which undergoes a series of modifications, including deoxygenation and amination.
Initial Steps[edit]

The biosynthesis pathway starts with the conversion of a common sugar nucleotide, such as UDP-glucose, into a deoxygenated form. This is achieved through the action of specific dehydratase enzymes that remove hydroxyl groups.
Amination and Final Modifications[edit]

Subsequent steps involve the introduction of an amino group, typically through the action of transaminase enzymes. The final structure of vancosamine is achieved after several more modifications, including the addition of methyl groups and further deoxygenation.
Role in Antibiotics[edit]
Vancosamine is a critical component of the glycopeptide antibiotic vancomycin. It enhances the antibiotic's ability to bind to the D-Ala-D-Ala terminus of bacterial cell wall precursors, thereby inhibiting cell wall synthesis and exerting its antibacterial effects. This makes vancomycin an important treatment option for infections caused by Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA).
Related Pages[edit]
-
Vancosamine and Epivancosamine
-
Vancosamine Biosynthesis Part 1
-
Vancosamine Biosynthesis Part 2