Chromic acid: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 34: | Line 34: | ||
[[Category:Oxidizing agents]] | [[Category:Oxidizing agents]] | ||
{{Chem-stub}} | {{Chem-stub}} | ||
<gallery> | |||
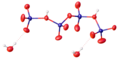
File:ChromicAcid.svg|Chromic acid structure | |||
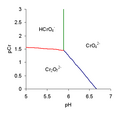
File:Predominance_diagram_Cr.png|Predominance diagram of chromium species | |||
File:290300-ICSD.png|Chromic acid | |||
</gallery> | |||
Latest revision as of 01:58, 18 February 2025
Chromic Acid
Chromic acid is an oxyacid of chromium and a powerful oxidizing agent. It is a strong acid that can be found in a number of different forms, including a monohydrate and an anhydrous form.
Chemical Structure[edit]
Chromic acid features a chromium atom in the center, surrounded by four oxygen atoms. The chromium atom is in the +6 oxidation state. The chemical formula for chromic acid is H2CrO4.
Preparation[edit]
Chromic acid is typically prepared by adding concentrated sulfuric acid to a dichromate, such as potassium dichromate. The reaction produces chromic acid and a sulfate salt.
Uses[edit]
Chromic acid is used in a variety of applications. It is commonly used in chromium plating, where it is used to coat metals with a thin layer of chromium to prevent corrosion. It is also used in the production of chromates and dichromates for use in pigments and corrosion inhibitors. In addition, chromic acid is used as a cleaning agent for glassware in laboratories.
Safety[edit]
Chromic acid is a highly corrosive substance and can cause severe burns and eye damage. It is also a strong oxidizer and can cause or intensify fires. In addition, chromic acid is harmful if inhaled and can cause respiratory irritation.
Environmental Impact[edit]
Chromic acid can have a significant environmental impact. It is toxic to aquatic life and can cause long-term adverse effects in the aquatic environment. It can also contaminate soil and groundwater if not properly disposed of.
See Also[edit]
-
Chromic acid structure
-
Predominance diagram of chromium species
-
Chromic acid