Terpenoid: Difference between revisions
CSV import |
CSV import |
||
| Line 32: | Line 32: | ||
[[Category:Herbal remedies]] | [[Category:Herbal remedies]] | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:Taxol.svg|Taxol | |||
File:Terpineol_alpha.svg|Alpha-Terpineol | |||
File:(S)-Humulone.svg|Humulone | |||
File:All-trans-Retinol2.svg|All-trans-Retinol | |||
File:Beta-thujaplicin.png|Beta-Thujaplicin | |||
File:Limonin.svg|Limonin | |||
File:Geosmin_Structural_Formulae.svg|Geosmin | |||
</gallery> | |||
Latest revision as of 10:56, 18 February 2025
Terpenoids, also known as isoprenoids, are a large and diverse class of naturally occurring organic chemicals derived from the five-carbon compound isoprene. They are found in all classes of living things and are the largest group of natural products. Terpenoids are used in traditional herbal remedies, and are under investigation for antibacterial, antineoplastic, and other pharmaceutical functions.
Structure and biosynthesis[edit]
Terpenoids are derived biosynthetically from units of isopentenyl pyrophosphate. They are classified according to the number of isoprenoid units in the molecule. A prefix in the name indicates the number of terpene units needed to assemble the molecule. "Mono", "di", "tri", "sesqui", "sester", "tri", "tetra", and "poly" refer to 1, 2, 3, 1.5, 2.5, 3, 4, and many terpene units, respectively.
Classification[edit]
Terpenoids can be classified into several categories based on the number of isoprene units present. These include monoterpenoids, sesquiterpenoids, diterpenoids, triterpenoids, and tetraterpenoids. Each of these classes is further divided into groups based on structural similarities and biosynthetic pathways.
Uses and applications[edit]
Terpenoids have a wide range of uses and applications. They are widely used in traditional herbal remedies and are under investigation for antibacterial, antineoplastic, and other pharmaceutical functions. Terpenoids are also used in organic chemistry as synthetic intermediates for chemical synthesis.
See also[edit]
References[edit]
<references group="" responsive="1"></references>
-
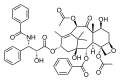
Taxol
-
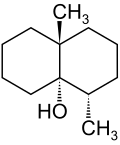
Alpha-Terpineol
-
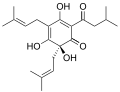
Humulone
-
All-trans-Retinol
-
Beta-Thujaplicin
-
Limonin
-
Geosmin