Tropanserin: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Tropanserin == | |||
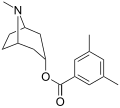
[[File:Tropanserin.svg|thumb|Chemical structure of Tropanserin]] | |||
Tropanserin | |||
'''Tropanserin''' is a chemical compound that acts as a selective [[serotonin receptor]] antagonist. It is primarily used in scientific research to study the role of serotonin in various physiological processes. | |||
Tropanserin acts as a | |||
== | == Chemical Properties == | ||
== | Tropanserin is classified as a [[serotonin antagonist]], meaning it inhibits the action of serotonin by blocking its receptors. The chemical structure of Tropanserin is characterized by a tropane ring, which is a bicyclic structure common to many alkaloids. | ||
== Mechanism of Action == | |||
Tropanserin functions by binding to serotonin receptors, particularly the 5-HT<sub>3</sub> receptor subtype. By blocking these receptors, Tropanserin can modulate the effects of serotonin in the [[central nervous system]] and [[peripheral nervous system]]. This makes it a valuable tool in research focused on understanding serotonin's role in mood regulation, anxiety, and other neurological functions. | |||
== Synthesis == | |||
[[File:Tropanserin_synthesis.svg|thumb|Synthesis pathway of Tropanserin]] | |||
The synthesis of Tropanserin involves several chemical reactions starting from basic organic compounds. The process typically includes the formation of the tropane ring followed by the addition of functional groups that confer its receptor-binding properties. Detailed synthetic pathways are often proprietary, but general methods involve standard organic synthesis techniques. | |||
== Applications in Research == | |||
Tropanserin is used extensively in [[pharmacological]] studies to investigate the effects of serotonin receptor antagonism. It helps researchers understand the potential therapeutic applications of serotonin modulation, including the treatment of [[depression]], [[anxiety disorders]], and [[gastrointestinal disorders]]. | |||
== Related Compounds == | |||
Tropanserin is part of a broader class of compounds known as tropane alkaloids, which include other well-known substances such as [[cocaine]] and [[atropine]]. These compounds share a similar chemical structure but differ in their pharmacological effects. | |||
== Related Pages == | |||
* [[Serotonin receptor]] | |||
* [[Serotonin antagonist]] | * [[Serotonin antagonist]] | ||
* [[ | * [[Tropane alkaloid]] | ||
== References == | |||
{{Reflist}} | |||
[[Category:Serotonin antagonists]] | [[Category:Serotonin antagonists]] | ||
[[Category: | [[Category:Tropane alkaloids]] | ||
<gallery> | |||
File:Tropanserin.svg|Structure of Tropanserin | |||
File:Tropanserin_synthesis.svg|Synthesis of Tropanserin | |||
</gallery> | |||
Latest revision as of 01:52, 17 February 2025
Tropanserin[edit]

Tropanserin is a chemical compound that acts as a selective serotonin receptor antagonist. It is primarily used in scientific research to study the role of serotonin in various physiological processes.
Chemical Properties[edit]
Tropanserin is classified as a serotonin antagonist, meaning it inhibits the action of serotonin by blocking its receptors. The chemical structure of Tropanserin is characterized by a tropane ring, which is a bicyclic structure common to many alkaloids.
Mechanism of Action[edit]
Tropanserin functions by binding to serotonin receptors, particularly the 5-HT3 receptor subtype. By blocking these receptors, Tropanserin can modulate the effects of serotonin in the central nervous system and peripheral nervous system. This makes it a valuable tool in research focused on understanding serotonin's role in mood regulation, anxiety, and other neurological functions.
Synthesis[edit]

The synthesis of Tropanserin involves several chemical reactions starting from basic organic compounds. The process typically includes the formation of the tropane ring followed by the addition of functional groups that confer its receptor-binding properties. Detailed synthetic pathways are often proprietary, but general methods involve standard organic synthesis techniques.
Applications in Research[edit]
Tropanserin is used extensively in pharmacological studies to investigate the effects of serotonin receptor antagonism. It helps researchers understand the potential therapeutic applications of serotonin modulation, including the treatment of depression, anxiety disorders, and gastrointestinal disorders.
Related Compounds[edit]
Tropanserin is part of a broader class of compounds known as tropane alkaloids, which include other well-known substances such as cocaine and atropine. These compounds share a similar chemical structure but differ in their pharmacological effects.
Related Pages[edit]
References[edit]
<references group="" responsive="1"></references>
-
Structure of Tropanserin
-
Synthesis of Tropanserin