Carbol fuchsin: Difference between revisions
CSV import |
CSV import |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''Carbol | '''Carbol Fuchsin''' is a complex organic compound used primarily as a staining agent in microbiology. It is a mixture of phenol and basic fuchsin, which is a magenta dye. This compound is particularly important in the field of bacteriology for its role in the [[Ziehl-Neelsen stain]], a technique used to identify [[acid-fast bacteria]], such as [[Mycobacterium tuberculosis]]. | ||
== Composition == | == Composition and Properties == | ||
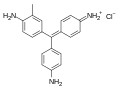
Carbol Fuchsin is composed of basic fuchsin, which is a mixture of rosaniline, pararosaniline, and magenta II, dissolved in phenol. The phenol acts as a mordant, enhancing the dye's ability to penetrate the waxy cell walls of acid-fast bacteria. The chemical structure of rosaniline hydrochloride, a component of basic fuchsin, is depicted in the image to the right. | |||
Carbol fuchsin is | |||
The dye is known for its vibrant magenta color and its ability to bind strongly to cellular components, making it an effective stain for microscopic examination. | |||
== | == Applications in Microbiology == | ||
Carbol Fuchsin is most famously used in the [[Ziehl-Neelsen stain]], a differential staining technique that distinguishes acid-fast organisms from non-acid-fast organisms. In this procedure, the specimen is first stained with Carbol Fuchsin, then decolorized with an acid-alcohol solution, and finally counterstained with methylene blue or malachite green. | |||
== | The acid-fast bacteria retain the Carbol Fuchsin stain due to the high lipid content in their cell walls, which resists decolorization. This property is crucial for the identification of [[Mycobacterium]] species, including the causative agents of [[tuberculosis]] and [[leprosy]]. | ||
Carbol fuchsin | |||
== Preparation and Handling == | |||
Carbol Fuchsin is prepared by dissolving basic fuchsin in a solution of phenol and water. The preparation must be handled with care, as phenol is a toxic compound. Proper laboratory safety protocols, including the use of gloves and eye protection, should be followed when preparing and using Carbol Fuchsin. | |||
== Related Pages == | |||
* [[Ziehl-Neelsen stain]] | * [[Ziehl-Neelsen stain]] | ||
* [[Acid-fast | * [[Acid-fast bacteria]] | ||
* [[ | * [[Mycobacterium tuberculosis]] | ||
* [[ | * [[Phenol]] | ||
* [[Basic fuchsin]] | |||
[[ | |||
[[Category:Microbiology]] | |||
[[Category:Staining dyes]] | |||
<gallery> | |||
File:Rosaniline_hydrochloride.svg|Rosaniline hydrochloride | |||
</gallery> | |||
Latest revision as of 21:00, 25 February 2025
Carbol Fuchsin is a complex organic compound used primarily as a staining agent in microbiology. It is a mixture of phenol and basic fuchsin, which is a magenta dye. This compound is particularly important in the field of bacteriology for its role in the Ziehl-Neelsen stain, a technique used to identify acid-fast bacteria, such as Mycobacterium tuberculosis.
Composition and Properties[edit]
Carbol Fuchsin is composed of basic fuchsin, which is a mixture of rosaniline, pararosaniline, and magenta II, dissolved in phenol. The phenol acts as a mordant, enhancing the dye's ability to penetrate the waxy cell walls of acid-fast bacteria. The chemical structure of rosaniline hydrochloride, a component of basic fuchsin, is depicted in the image to the right.
The dye is known for its vibrant magenta color and its ability to bind strongly to cellular components, making it an effective stain for microscopic examination.
Applications in Microbiology[edit]
Carbol Fuchsin is most famously used in the Ziehl-Neelsen stain, a differential staining technique that distinguishes acid-fast organisms from non-acid-fast organisms. In this procedure, the specimen is first stained with Carbol Fuchsin, then decolorized with an acid-alcohol solution, and finally counterstained with methylene blue or malachite green.
The acid-fast bacteria retain the Carbol Fuchsin stain due to the high lipid content in their cell walls, which resists decolorization. This property is crucial for the identification of Mycobacterium species, including the causative agents of tuberculosis and leprosy.
Preparation and Handling[edit]
Carbol Fuchsin is prepared by dissolving basic fuchsin in a solution of phenol and water. The preparation must be handled with care, as phenol is a toxic compound. Proper laboratory safety protocols, including the use of gloves and eye protection, should be followed when preparing and using Carbol Fuchsin.
Related Pages[edit]
-
Rosaniline hydrochloride