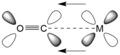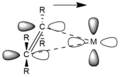Pi backbonding: Difference between revisions
CSV import |
CSV import |
||
| Line 28: | Line 28: | ||
{{stub}} | {{stub}} | ||
<gallery> | |||
File:CO-M_σ_bonding.png|CO-M σ Bonding | |||
File:CO-M_Pi_Backbond.png|CO-M Pi Backbonding | |||
File:Alkene-M_Sigma_Bond.png|Alkene-M Sigma Bond | |||
File:Alkene-M_Pi_Backbond.png|Alkene-M Pi Backbonding | |||
File:Connelly-Orpen-R3P-M-sigma-bonding.png|Connelly-Orpen R3P-M Sigma Bonding | |||
File:Connelly-Orpen-R3P-M-pi-backbonding.png|Connelly-Orpen R3P-M Pi Backbonding | |||
</gallery> | |||
Latest revision as of 11:11, 18 February 2025
Pi backbonding also known as π backbonding is a concept in chemistry that involves the interaction between the filled π orbital of a ligand and an empty orbital of a transition metal. The term "backbonding" is a reference to the donation of electrons from the metal to the ligand, which is a reversal of the usual direction of electron flow.
Etymology[edit]
The term "Pi backbonding" is derived from the Greek letter "π" (pi), which is used in chemistry to denote one of the types of molecular orbitals. The "backbonding" part of the term refers to the unusual direction of electron flow from the metal to the ligand.
Concept[edit]
Pi backbonding is a type of chemical bonding that occurs between a transition metal and a ligand. In most cases, the ligand has a pair of non-bonding electrons that it can donate to the metal. However, in pi backbonding, the metal donates a pair of its own electrons to the ligand. This donation of electrons from the metal to the ligand is what gives rise to the term "backbonding".
The concept of pi backbonding is particularly important in the chemistry of transition metals. It helps to explain the behavior of many transition metal complexes, including their spectroscopic properties and reactivity.