Pyruvate carboxylase: Difference between revisions
CSV import |
CSV import |
||
| Line 35: | Line 35: | ||
[[Category:Fatty Acid Synthesis]] | [[Category:Fatty Acid Synthesis]] | ||
[[Category:Genetic Disorders]] | [[Category:Genetic Disorders]] | ||
== Pyruvate_carboxylase == | |||
<gallery> | |||
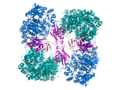
File:Pyruvate_Carboxylase_fromPDB_2QF7.png|Pyruvate Carboxylase from PDB 2QF7 | |||
File:Pyruvic-acid-2D-skeletal.png|Pyruvic acid 2D skeletal | |||
File:Oxaloacetic_acid.svg|Oxaloacetic acid | |||
File:Pyruvate_Carboxylase_2QF7,_sswilson7.png|Pyruvate Carboxylase 2QF7, sswilson7 | |||
File:Pyruvate_carboxylase_3HO8,_sswilson7.png|Pyruvate carboxylase 3HO8, sswilson7 | |||
File:Mechanism_of_Pyruvate_Carboxylase,_5-15-2010,_sswilson7.png|Mechanism of Pyruvate Carboxylase, 5-15-2010, sswilson7 | |||
</gallery> | |||
Latest revision as of 00:51, 27 February 2025
Pyruvate Carboxylase[edit]
Pyruvate carboxylase is an essential enzyme involved in the process of gluconeogenesis, which is the production of glucose from non-carbohydrate sources. It is also involved in the biosynthesis of fatty acids. Pyruvate carboxylase catalyzes the carboxylation of pyruvate to form oxaloacetate, an important intermediate in these metabolic pathways.
Structure[edit]
Pyruvate carboxylase is a biotin-dependent enzyme, meaning it requires the presence of the vitamin biotin for its activity. It exists as a tetramer, with each subunit consisting of multiple domains. The enzyme contains a biotin carboxylase domain, a carboxyltransferase domain, and a biotin carboxyl carrier protein domain. These domains work together to facilitate the carboxylation reaction.
Function[edit]
The primary function of pyruvate carboxylase is to convert pyruvate, a three-carbon molecule, into oxaloacetate, a four-carbon molecule. This reaction is an important step in gluconeogenesis, as oxaloacetate can be converted into glucose. Pyruvate carboxylase is also involved in the synthesis of fatty acids, as oxaloacetate can be further converted into malate, which serves as a precursor for fatty acid synthesis.
Regulation[edit]
The activity of pyruvate carboxylase is tightly regulated to ensure proper metabolic control. The enzyme is allosterically activated by acetyl-CoA, a molecule that indicates the presence of excess energy in the cell. Acetyl-CoA binds to the enzyme and enhances its activity, promoting the conversion of pyruvate into oxaloacetate. Additionally, pyruvate carboxylase is regulated by phosphorylation, with phosphorylation inhibiting its activity.
Clinical Significance[edit]
Mutations in the gene encoding pyruvate carboxylase can lead to a rare genetic disorder known as pyruvate carboxylase deficiency. This condition is characterized by impaired gluconeogenesis and fatty acid synthesis, resulting in a variety of symptoms including developmental delay, hypotonia, and metabolic acidosis. Treatment for pyruvate carboxylase deficiency typically involves dietary modifications and supplementation with specific nutrients.
See Also[edit]
References[edit]
<references />
Pyruvate_carboxylase[edit]
-
Pyruvate Carboxylase from PDB 2QF7
-
Pyruvic acid 2D skeletal
-
Oxaloacetic acid
-
Pyruvate Carboxylase 2QF7, sswilson7
-
Pyruvate carboxylase 3HO8, sswilson7
-
Mechanism of Pyruvate Carboxylase, 5-15-2010, sswilson7