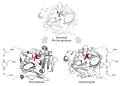Catalytic triad: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 77: | Line 77: | ||
[[Category:Enzyme Purification]] | [[Category:Enzyme Purification]] | ||
[[Category:Enzyme Characterization]] | [[Category:Enzyme Characterization]] | ||
<gallery> | |||
File:Catalytic_triad_of_TEV_protease.png|Catalytic triad of TEV protease | |||
File:Triad_chemical_mech.png|Triad chemical mechanism | |||
File:Triads_and_their_substrates_annotated.svg|Triads and their substrates annotated | |||
File:Triad_divergence.svg|Triad divergence | |||
File:Triad_convergence_ser_cys.svg|Triad convergence serine and cysteine | |||
File:Triad_convergence_thr.svg|Triad convergence threonine | |||
</gallery> | |||
Latest revision as of 11:01, 18 February 2025
Catalytic Triad[edit]
The catalytic triad is a key component of enzymatic activity, particularly in serine proteases. It consists of three amino acid residues that work together to facilitate the catalytic function of the enzyme. The catalytic triad is composed of serine, histidine, and aspartate residues, which are strategically positioned within the enzyme's active site.
Structure[edit]
The catalytic triad is typically found in a specific arrangement within the active site of serine proteases. The serine residue is located at the center of the triad, with the histidine and aspartate residues positioned on either side. This arrangement allows for efficient catalysis by promoting the formation of a reactive intermediate.
Function[edit]
The catalytic triad plays a crucial role in the enzymatic activity of serine proteases. The serine residue acts as a nucleophile, attacking the peptide bond of the substrate molecule. This results in the formation of a covalent intermediate between the serine residue and the substrate.
The histidine residue acts as a general base, abstracting a proton from the serine hydroxyl group. This deprotonation enhances the nucleophilicity of the serine residue, allowing it to efficiently attack the peptide bond.
The aspartate residue, on the other hand, acts as a general acid, donating a proton to the leaving group. This protonation facilitates the cleavage of the peptide bond and the release of the product.
Importance[edit]
The catalytic triad is essential for the proper functioning of serine proteases. These enzymes are involved in a wide range of biological processes, including digestion, blood clotting, and immune response. Without the catalytic triad, the enzymatic activity of serine proteases would be significantly impaired, leading to various physiological abnormalities.
Examples[edit]
Several well-known enzymes contain the catalytic triad, including chymotrypsin, trypsin, and elastase. These enzymes are responsible for the hydrolysis of peptide bonds and are crucial for protein digestion in the gastrointestinal tract.
References[edit]
<references />
See Also[edit]
-
Catalytic triad of TEV protease
-
Triad chemical mechanism
-
Triads and their substrates annotated
-
Triad divergence
-
Triad convergence serine and cysteine
-
Triad convergence threonine
- Enzymes
- Proteins
- Biochemistry
- Catalysis
- Enzyme Kinetics
- Enzyme Mechanisms
- Enzyme Inhibition
- Enzyme Regulation
- Enzyme Structure
- Enzyme Classification
- Enzyme Nomenclature
- Enzyme Assays
- Enzyme Engineering
- Enzyme Immobilization
- Enzyme Catalysis
- Enzyme Substrates
- Enzyme Products
- Enzyme Specificity
- Enzyme Evolution
- Enzyme Function
- Enzyme Localization
- Enzyme Expression
- Enzyme Purification
- Enzyme Characterization