Carcinoid syndrome: Difference between revisions
No edit summary Tag: visualeditor-wikitext |
CSV import |
||
| Line 1: | Line 1: | ||
{{SI}} | |||
{{Infobox medical condition | |||
| name = Carcinoid syndrome | |||
| image = [[File:Carcinoid_syndrome_presentation.svg|250px]] | |||
| caption = Presentation of carcinoid syndrome | |||
| field = [[Oncology]] | |||
| symptoms = [[Flushing]], [[diarrhea]], [[wheezing]], [[abdominal pain]] | |||
| complications = [[Heart valve disease]], [[pellagra]] | |||
| onset = Usually in the 5th to 7th decade of life | |||
| duration = Chronic | |||
| causes = [[Neuroendocrine tumors]] | |||
| risks = [[Metastatic disease]], [[liver metastases]] | |||
| diagnosis = [[Urinary 5-HIAA]], [[chromogranin A]], [[imaging studies]] | |||
| differential = [[Irritable bowel syndrome]], [[menopausal flushing]], [[asthma]] | |||
| prevention = None | |||
| treatment = [[Surgical resection]], [[somatostatin analogs]], [[interferon therapy]] | |||
| medication = [[Octreotide]], [[lanreotide]] | |||
| prognosis = Variable, depends on extent of disease | |||
| frequency = Rare | |||
}} | |||
'''Other Names: ''' | '''Other Names: ''' | ||
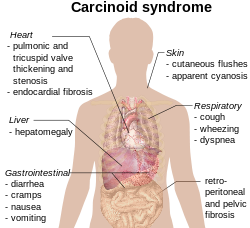
Carcinoid syndrome refers to a group of symptoms that are associated with [[carcinoid tumors]] (rare, slow-growing tumors that occur most frequently in the [[gastroinestinal tract]] or lungs). | Carcinoid syndrome refers to a group of symptoms that are associated with [[carcinoid tumors]] (rare, slow-growing tumors that occur most frequently in the [[gastroinestinal tract]] or lungs). | ||
=='''Symptoms'''== | =='''Symptoms'''== | ||
Affected people may experience skin [[flushing]], abdominal pain, [[diarrhea]], difficulty breathing, [[rapid heart rate]], low [[blood pressure]], skin [[lesions on the face]] (telangiectasias), and wheezing. | Affected people may experience skin [[flushing]], abdominal pain, [[diarrhea]], difficulty breathing, [[rapid heart rate]], low [[blood pressure]], skin [[lesions on the face]] (telangiectasias), and wheezing. | ||
In later stages, carcinoid syndrome may damage the heart valves, resulting in symptoms of [[congestive heart failure]]. | In later stages, carcinoid syndrome may damage the heart valves, resulting in symptoms of [[congestive heart failure]]. | ||
<youtube> | <youtube> | ||
title='''{{PAGENAME}}''' | title='''{{PAGENAME}}''' | ||
| Line 20: | Line 34: | ||
height=600 | height=600 | ||
</youtube> | </youtube> | ||
=='''Cause'''== | =='''Cause'''== | ||
The condition occurs when the carcinoid tumor secretes [[serotonin]] or other chemicals into the bloodstream. Only 10% of people with [[carcinoid tumors]] develop carcinoid syndrome; most have advanced stage carcinoid tumors that have spread to the liver. | The condition occurs when the carcinoid tumor secretes [[serotonin]] or other chemicals into the bloodstream. Only 10% of people with [[carcinoid tumors]] develop carcinoid syndrome; most have advanced stage carcinoid tumors that have spread to the liver. | ||
==Diagnosis== | ==Diagnosis== | ||
With a certain degree of clinical suspicion, the most useful initial test is the 24-hour [[urine]] levels of [[5-HIAA]] (5-hydroxyindoleacetic acid), the end product of serotonin metabolism. | With a certain degree of clinical suspicion, the most useful initial test is the 24-hour [[urine]] levels of [[5-HIAA]] (5-hydroxyindoleacetic acid), the end product of serotonin metabolism. | ||
Patients with carcinoid syndrome usually excrete more than 25 mg of 5-HIAA per day. | Patients with carcinoid syndrome usually excrete more than 25 mg of 5-HIAA per day. | ||
==Imaging== | ==Imaging== | ||
Usually, on a CT scan, a spider-like/crab-like change is visible in the mesentery due to the fibrosis from the release of serotonin. [[Fludeoxyglucose (18F)|<sup>8</sup>F-FDG]] PET/CT, which evaluate for increased metabolism of glucose, may also aid in localizing the carcinoid lesion or evaluating for metastases. | Usually, on a CT scan, a spider-like/crab-like change is visible in the mesentery due to the fibrosis from the release of serotonin. [[Fludeoxyglucose (18F)|<sup>8</sup>F-FDG]] PET/CT, which evaluate for increased metabolism of glucose, may also aid in localizing the carcinoid lesion or evaluating for metastases. | ||
[[Chromogranin A|hromogranin A]] and [[serotonin|platelets serotonin]] are increased. | [[Chromogranin A|hromogranin A]] and [[serotonin|platelets serotonin]] are increased. | ||
==Prognosis== | ==Prognosis== | ||
[[Prognosis]] varies from individual to individual. It ranges from a 95% 5-year survival for localized disease to an 80% 5-year survival for those with liver metastases.The average survival time from the start of octreotide treatment has increased to about 12 years. | [[Prognosis]] varies from individual to individual. It ranges from a 95% 5-year survival for localized disease to an 80% 5-year survival for those with liver metastases.The average survival time from the start of octreotide treatment has increased to about 12 years. | ||
| Line 37: | Line 47: | ||
Treatment generally involves addressing the underlying carcinoid tumor and medications to alleviate symptoms. | Treatment generally involves addressing the underlying carcinoid tumor and medications to alleviate symptoms. | ||
The medication(s) listed below have been approved by the Food and Drug Administration (FDA) as orphan products for treatment of this condition. | The medication(s) listed below have been approved by the Food and Drug Administration (FDA) as orphan products for treatment of this condition. | ||
*'''[[Octreotide]]''' (Brand name: Sandostatin LAR)Reduction of growth hormone and IGF-1 (somatomedin C) in acromegaly. | *'''[[Octreotide]]''' (Brand name: Sandostatin LAR)Reduction of growth hormone and IGF-1 (somatomedin C) in acromegaly. | ||
*[[Telotristat etiprate|'''Telotristat etiprate''']] (Brand name: Xermelo) Treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (SSA) therapy in adults inadequately controlled by SSA therapy. | *[[Telotristat etiprate|'''Telotristat etiprate''']] (Brand name: Xermelo) Treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (SSA) therapy in adults inadequately controlled by SSA therapy. | ||
[[Surgical]] resection of tumor and [[chemotherapy]] ([[5-FU]] and [[doxorubicin]]) | [[Surgical]] resection of tumor and [[chemotherapy]] ([[5-FU]] and [[doxorubicin]]) | ||
{{stub}} | {{stub}} | ||
Latest revision as of 00:45, 6 April 2025

Editor-In-Chief: Prab R Tumpati, MD
Obesity, Sleep & Internal medicine
Founder, WikiMD Wellnesspedia &
W8MD medical weight loss NYC and sleep center NYC
| Carcinoid syndrome | |
|---|---|
| |
| Synonyms | N/A |
| Pronounce | N/A |
| Specialty | N/A |
| Symptoms | Flushing, diarrhea, wheezing, abdominal pain |
| Complications | Heart valve disease, pellagra |
| Onset | Usually in the 5th to 7th decade of life |
| Duration | Chronic |
| Types | N/A |
| Causes | Neuroendocrine tumors |
| Risks | Metastatic disease, liver metastases |
| Diagnosis | Urinary 5-HIAA, chromogranin A, imaging studies |
| Differential diagnosis | Irritable bowel syndrome, menopausal flushing, asthma |
| Prevention | None |
| Treatment | Surgical resection, somatostatin analogs, interferon therapy |
| Medication | Octreotide, lanreotide |
| Prognosis | Variable, depends on extent of disease |
| Frequency | Rare |
| Deaths | N/A |
Other Names:
Carcinoid syndrome refers to a group of symptoms that are associated with carcinoid tumors (rare, slow-growing tumors that occur most frequently in the gastroinestinal tract or lungs).
Symptoms[edit]
Affected people may experience skin flushing, abdominal pain, diarrhea, difficulty breathing, rapid heart rate, low blood pressure, skin lesions on the face (telangiectasias), and wheezing. In later stages, carcinoid syndrome may damage the heart valves, resulting in symptoms of congestive heart failure.
Cause[edit]
The condition occurs when the carcinoid tumor secretes serotonin or other chemicals into the bloodstream. Only 10% of people with carcinoid tumors develop carcinoid syndrome; most have advanced stage carcinoid tumors that have spread to the liver.
Diagnosis[edit]
With a certain degree of clinical suspicion, the most useful initial test is the 24-hour urine levels of 5-HIAA (5-hydroxyindoleacetic acid), the end product of serotonin metabolism. Patients with carcinoid syndrome usually excrete more than 25 mg of 5-HIAA per day.
Imaging[edit]
Usually, on a CT scan, a spider-like/crab-like change is visible in the mesentery due to the fibrosis from the release of serotonin. 8F-FDG PET/CT, which evaluate for increased metabolism of glucose, may also aid in localizing the carcinoid lesion or evaluating for metastases. hromogranin A and platelets serotonin are increased.
Prognosis[edit]
Prognosis varies from individual to individual. It ranges from a 95% 5-year survival for localized disease to an 80% 5-year survival for those with liver metastases.The average survival time from the start of octreotide treatment has increased to about 12 years.
Treatment[edit]
Treatment generally involves addressing the underlying carcinoid tumor and medications to alleviate symptoms. The medication(s) listed below have been approved by the Food and Drug Administration (FDA) as orphan products for treatment of this condition.
- Octreotide (Brand name: Sandostatin LAR)Reduction of growth hormone and IGF-1 (somatomedin C) in acromegaly.
- Telotristat etiprate (Brand name: Xermelo) Treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (SSA) therapy in adults inadequately controlled by SSA therapy.
Surgical resection of tumor and chemotherapy (5-FU and doxorubicin)
|
|
|
This template is no longer used; please see Template:Endocrine pathology for a suitable replacement
NIH genetic and rare disease info[edit]
Carcinoid syndrome is a rare disease.
| Rare and genetic diseases | ||||||
|---|---|---|---|---|---|---|
|
Rare diseases - Carcinoid syndrome
|


