Mipafox: Difference between revisions
CSV import |
CSV import |
||
| Line 23: | Line 23: | ||
{{Chemical-compound-stub}} | {{Chemical-compound-stub}} | ||
{{Toxicology-stub}} | {{Toxicology-stub}} | ||
<gallery> | |||
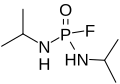
File:Mipafox.svg|Mipafox | |||
</gallery> | |||
Latest revision as of 05:12, 3 March 2025
Mipafox is a highly toxic organophosphate compound that is used as a pesticide. It is known for its potent neurotoxicity and is classified as a nerve gas in chemical warfare.
History[edit]
Mipafox was first synthesized in the 1940s as part of a series of organophosphates developed for potential use in agriculture. However, due to its high toxicity, it was never widely used for this purpose. Instead, it found use in laboratory settings as a tool for studying the effects of nerve gases on the body.
Chemical Properties[edit]
Mipafox is a colorless, odorless liquid at room temperature. It is soluble in water and most organic solvents. The chemical formula for Mipafox is C11H16NO2PS. It is an organophosphate, meaning it contains a phosphorus atom bonded to an oxygen atom and to carbon atoms. This class of compounds is known for their ability to inhibit the enzyme acetylcholinesterase, which is crucial for nerve function.
Toxicity[edit]
Mipafox is highly toxic, with a lethal dose for humans estimated to be less than 1 milligram per kilogram of body weight. Exposure can occur through inhalation, ingestion, or skin contact. Symptoms of exposure include difficulty breathing, blurred vision, vomiting, and seizures. In severe cases, exposure can lead to death due to respiratory failure.
Treatment[edit]
Treatment for Mipafox poisoning involves immediate removal from the source of exposure and supportive care to manage symptoms. Antidotes such as pralidoxime and atropine can be used to counteract the effects of the poison. However, these treatments are most effective if administered soon after exposure.
See Also[edit]
This article is a Chemical compound-related stub. You can help WikiMD by expanding it!

This article is a toxicology-related stub. You can help WikiMD by expanding it!
-
Mipafox