Carboxypeptidase B2: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''Carboxypeptidase B2''' ( | {{Short description|An enzyme involved in the regulation of fibrinolysis}} | ||
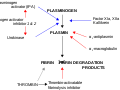
'''Carboxypeptidase B2''' (CPB2), also known as plasma carboxypeptidase B, is an enzyme that plays a crucial role in the regulation of the [[fibrinolysis]] process. It is a member of the [[carboxypeptidase]] family of enzymes, which are responsible for cleaving the C-terminal amino acids from proteins and peptides. | |||
== | === Structure === | ||
Carboxypeptidase B2 is a | Carboxypeptidase B2 is a zinc-dependent metalloenzyme. It is synthesized in the liver and circulates in the plasma as an inactive zymogen known as procarboxypeptidase B2. Upon activation, it becomes an active enzyme capable of modulating fibrinolysis. | ||
== | === Function === | ||
The primary function of Carboxypeptidase B2 is to regulate fibrinolysis by removing C-terminal lysine residues from partially degraded fibrin. This action reduces the binding affinity of [[plasminogen]] to fibrin, thereby slowing down the conversion of plasminogen to [[plasmin]], the enzyme responsible for fibrin degradation. By doing so, CPB2 helps to stabilize the clot and prevent excessive bleeding. | |||
== | === Activation === | ||
Carboxypeptidase B2 is activated by the [[thrombin-thrombomodulin complex]] during the coagulation cascade. This activation is crucial for its role in modulating fibrinolysis and maintaining hemostatic balance. | |||
== | == Clinical Significance == | ||
Alterations in the activity of Carboxypeptidase B2 can lead to various clinical conditions. Overactivity of CPB2 can result in impaired fibrinolysis, leading to thrombosis, while underactivity can cause excessive bleeding due to enhanced fibrinolysis. | |||
== Related pages == | |||
* [[Fibrinolysis]] | * [[Fibrinolysis]] | ||
* [[ | * [[Plasminogen]] | ||
* [[Thrombin]] | |||
* [[Coagulation]] | |||
* [ | |||
* [ | |||
[[Category:Enzymes]] | [[Category:Enzymes]] | ||
[[Category: | [[Category:Coagulation system]] | ||
<gallery> | |||
File:Fibrinolysis.svg|Fibrinolysis | |||
</gallery> | |||
Latest revision as of 21:03, 25 February 2025
An enzyme involved in the regulation of fibrinolysis
Carboxypeptidase B2 (CPB2), also known as plasma carboxypeptidase B, is an enzyme that plays a crucial role in the regulation of the fibrinolysis process. It is a member of the carboxypeptidase family of enzymes, which are responsible for cleaving the C-terminal amino acids from proteins and peptides.
Structure[edit]
Carboxypeptidase B2 is a zinc-dependent metalloenzyme. It is synthesized in the liver and circulates in the plasma as an inactive zymogen known as procarboxypeptidase B2. Upon activation, it becomes an active enzyme capable of modulating fibrinolysis.
Function[edit]
The primary function of Carboxypeptidase B2 is to regulate fibrinolysis by removing C-terminal lysine residues from partially degraded fibrin. This action reduces the binding affinity of plasminogen to fibrin, thereby slowing down the conversion of plasminogen to plasmin, the enzyme responsible for fibrin degradation. By doing so, CPB2 helps to stabilize the clot and prevent excessive bleeding.
Activation[edit]
Carboxypeptidase B2 is activated by the thrombin-thrombomodulin complex during the coagulation cascade. This activation is crucial for its role in modulating fibrinolysis and maintaining hemostatic balance.
Clinical Significance[edit]
Alterations in the activity of Carboxypeptidase B2 can lead to various clinical conditions. Overactivity of CPB2 can result in impaired fibrinolysis, leading to thrombosis, while underactivity can cause excessive bleeding due to enhanced fibrinolysis.
Related pages[edit]
-
Fibrinolysis