Calcium nitrate: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''Calcium | {{Short description|Chemical compound}} | ||
{{Use dmy dates|date=October 2023}} | |||
==Calcium Nitrate== | |||
[[File:Calcium_nitrate.png|thumb|right|200px|Calcium nitrate powder]] | |||
'''Calcium nitrate''', also known as Norgessalpeter, is an inorganic compound with the formula Ca(NO₃)₂. This colorless salt absorbs moisture from the air and is commonly found as a tetrahydrate. It is mainly used as a component in fertilizers but has other applications as well. | |||
==Chemical Properties== | ==Chemical Properties== | ||
Calcium nitrate is a | [[File:Calcium-nitrate-3D-vdW.png|thumb|left|200px|3D model of calcium nitrate]] | ||
Calcium nitrate is a highly soluble compound in water and alcohol. It is a typical ionic nitrate, which means it can dissociate into calcium ions (Ca²⁺) and nitrate ions (NO₃⁻) in solution. The compound is hygroscopic, meaning it can absorb moisture from the environment, which is why it is often found in its hydrated form. | |||
==Production== | ==Production== | ||
Calcium nitrate is produced by | Calcium nitrate is produced by treating [[limestone]] with [[nitric acid]], followed by neutralization with [[ammonia]]. It can also be obtained as a byproduct of the [[Haber process]] for [[ammonia]] production. The reaction can be represented as: | ||
: CaCO₃ + 2 HNO₃ → Ca(NO₃)₂ + CO₂ + H₂O | |||
==Applications== | |||
[[File:Dusičnan_vápenatý.JPG|thumb|right|200px|Calcium nitrate in granular form]] | |||
== | ===Fertilizer=== | ||
Calcium nitrate is widely used as a fertilizer, providing both calcium and nitrogen to plants. It is especially beneficial for crops that require high levels of calcium, such as [[tomatoes]] and [[peppers]]. The compound helps improve fruit quality and shelf life. | |||
===Waste Water Treatment=== | ===Waste Water Treatment=== | ||
Calcium nitrate | [[File:Calcium_nitrate,_waste_water_treatment.jpg|thumb|left|200px|Calcium nitrate used in waste water treatment]] | ||
In waste water treatment, calcium nitrate is used to prevent the formation of [[hydrogen sulfide]] in sewage systems. It acts as an oxidizing agent, converting sulfides into sulfates, which are less odorous and corrosive. | |||
=== | ===Concrete and Construction=== | ||
Calcium nitrate is used in the construction industry as a set accelerator for [[concrete]]. It helps in reducing the setting time of concrete, especially in cold weather conditions, and improves the strength of the concrete. | |||
==Safety== | ==Safety and Handling== | ||
Calcium nitrate | Calcium nitrate should be handled with care, as it can cause irritation to the skin and eyes. It should be stored in a cool, dry place to prevent it from absorbing moisture and forming a solution. | ||
== | ==Related Pages== | ||
* [[ | * [[Calcium oxide]] | ||
* [[ | * [[Nitric acid]] | ||
* [[ | * [[Fertilizer]] | ||
* [[Concrete]] | |||
[[Category: | [[Category:Calcium compounds]] | ||
[[Category:Nitrates]] | [[Category:Nitrates]] | ||
[[Category:Fertilizers]] | [[Category:Fertilizers]] | ||
Latest revision as of 14:15, 21 February 2025
Chemical compound
Calcium Nitrate[edit]
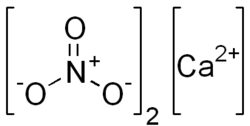
Calcium nitrate, also known as Norgessalpeter, is an inorganic compound with the formula Ca(NO₃)₂. This colorless salt absorbs moisture from the air and is commonly found as a tetrahydrate. It is mainly used as a component in fertilizers but has other applications as well.
Chemical Properties[edit]

Calcium nitrate is a highly soluble compound in water and alcohol. It is a typical ionic nitrate, which means it can dissociate into calcium ions (Ca²⁺) and nitrate ions (NO₃⁻) in solution. The compound is hygroscopic, meaning it can absorb moisture from the environment, which is why it is often found in its hydrated form.
Production[edit]
Calcium nitrate is produced by treating limestone with nitric acid, followed by neutralization with ammonia. It can also be obtained as a byproduct of the Haber process for ammonia production. The reaction can be represented as:
- CaCO₃ + 2 HNO₃ → Ca(NO₃)₂ + CO₂ + H₂O
Applications[edit]

Fertilizer[edit]
Calcium nitrate is widely used as a fertilizer, providing both calcium and nitrogen to plants. It is especially beneficial for crops that require high levels of calcium, such as tomatoes and peppers. The compound helps improve fruit quality and shelf life.
Waste Water Treatment[edit]

In waste water treatment, calcium nitrate is used to prevent the formation of hydrogen sulfide in sewage systems. It acts as an oxidizing agent, converting sulfides into sulfates, which are less odorous and corrosive.
Concrete and Construction[edit]
Calcium nitrate is used in the construction industry as a set accelerator for concrete. It helps in reducing the setting time of concrete, especially in cold weather conditions, and improves the strength of the concrete.
Safety and Handling[edit]
Calcium nitrate should be handled with care, as it can cause irritation to the skin and eyes. It should be stored in a cool, dry place to prevent it from absorbing moisture and forming a solution.