Seproxetine: Difference between revisions
CSV import |
CSV import |
||
| Line 20: | Line 20: | ||
{{stub}} | {{stub}} | ||
<gallery> | |||
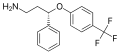
File:Seproxetine.svg|Seproxetine | |||
</gallery> | |||
Latest revision as of 01:41, 20 February 2025
Seproxetine is a serotonin reuptake inhibitor (SRI) that was being developed by Eli Lilly and Company for the treatment of depression. It is the active metabolite of fluoxetine, a selective serotonin reuptake inhibitor (SSRI). Seproxetine was being developed as a "me too" drug for the indication of depression and had reached phase II clinical trials before development was discontinued.
Pharmacology[edit]
Seproxetine, like its parent compound fluoxetine, works by inhibiting the reuptake of serotonin in the brain, thereby increasing levels of this neurotransmitter available to bind to the post-synaptic receptor. It has been shown to be a potent and selective inhibitor of serotonin reuptake, making it a powerful antidepressant.
Development and Clinical Trials[edit]
Seproxetine was being developed by Eli Lilly and Company, the same company that developed fluoxetine. It had reached phase II clinical trials for the treatment of depression. However, development was discontinued for unknown reasons. It is speculated that the development was stopped due to the emergence of unexpected side effects during the trials, or due to strategic decisions within the company.
See Also[edit]
References[edit]
<references />