Seviteronel: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 30: | Line 30: | ||
{{Pharmacology-stub}} | {{Pharmacology-stub}} | ||
{{Medicine-stub}} | {{Medicine-stub}} | ||
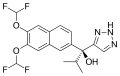
== Seviteronel == | |||
<gallery> | |||
File:VT-464.svg|Seviteronel | |||
</gallery> | |||
Latest revision as of 01:28, 20 February 2025
Seviteronel is an experimental drug that has been under investigation for its potential use in the treatment of certain types of cancer, notably those that are driven by hormone receptors, such as prostate cancer and breast cancer. It functions as a selective inhibitor of CYP17A1, an enzyme critical for the production of androgens and estrogens, which are hormones that can fuel the growth of hormone-sensitive cancers.
Mechanism of Action[edit]
Seviteronel inhibits the enzyme CYP17A1, also known as 17α-hydroxylase/17,20-lyase. CYP17A1 plays a pivotal role in the biosynthesis of androgens and estrogens, which are essential for the development and progression of certain cancers. By inhibiting this enzyme, seviteronel reduces the production of these hormones, thereby potentially slowing the growth of hormone-dependent tumors.
Clinical Trials[edit]
Seviteronel has been evaluated in various clinical trials for its efficacy and safety in treating cancers that are sensitive to hormone levels. These trials have primarily focused on prostate cancer and breast cancer, exploring the drug's impact on disease progression, survival rates, and quality of life among patients. The outcomes of these trials are crucial for determining the potential of seviteronel as a therapeutic option in oncology.
Potential Indications[edit]
The primary potential indications for seviteronel include:
- Prostate cancer, particularly castration-resistant prostate cancer (CRPC), which no longer responds to traditional hormone therapy.
- Breast cancer, especially types that express hormone receptors, such as estrogen receptor-positive (ER+) and progesterone receptor-positive (PR+) breast cancer.
Safety and Tolerability[edit]
The safety and tolerability profile of seviteronel is an important aspect of its clinical development. Adverse effects, drug interactions, and contraindications are closely monitored during clinical trials to ensure that seviteronel is safe for use in the target patient population.
Future Directions[edit]
Research on seviteronel continues to explore its potential across various types of hormone-sensitive cancers. Future studies may investigate its use in combination with other therapies, its efficacy in different stages of cancer, and its impact on overall survival and quality of life for patients.
See Also[edit]
Seviteronel[edit]
-
Seviteronel