N-Benzyl-2C-B: Difference between revisions
CSV import |
CSV import |
||
| Line 29: | Line 29: | ||
[[Category:Stimulants]] | [[Category:Stimulants]] | ||
{{pharmacology-stub}} | {{pharmacology-stub}} | ||
<gallery> | |||
File:25B-NB structure.png|N-Benzyl-2C-B | |||
</gallery> | |||
Latest revision as of 00:36, 20 February 2025
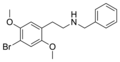
N-Benzyl-2C-B is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin. The full name of the chemical is 4-bromo-2,5-dimethoxy-N-benzylphenethylamine.
Chemistry[edit]
N-Benzyl-2C-B is a substituted phenethylamine featuring a phenyl ring bound to an amino (NH2) group through an ethyl chain. It has two methoxy groups CH3O- attached to carbons R2 and R5 as well as a bromine atom attached to carbon R4 of the phenyl ring. It is a benzyl analogue of 2C-B.
Pharmacology[edit]
The mechanism that produces the hallucinogenic and entheogenic effects of N-Benzyl-2C-B is most likely to result from action as a 5-HT2A serotonin receptor agonist in the brain, a mechanism of action shared by all of the hallucinogenic tryptamines and phenethylamines for which the mechanism of action is known.
Effects[edit]
The effects of N-Benzyl-2C-B are usually compared to those of 2C-B and LSD. It is known to produce a mixture of psychedelic, hallucinogenic and stimulant effects that include open and closed eye visuals, time distortion, enhanced introspection, increased empathy and euphoria.
Toxicity[edit]
The toxicity and long-term health effects of recreational N-Benzyl-2C-B use do not seem to have been studied in any scientific context and the exact toxic dosage is unknown.
Legality[edit]
N-Benzyl-2C-B is currently a gray area compound within many parts of the world. This means that it is not known to be specifically illegal within most countries, but people may still be charged for its possession under certain circumstances such as under analogue laws and with the intent to sell or consume.
See also[edit]
References[edit]
<references />
-
N-Benzyl-2C-B