Aldol reaction: Difference between revisions
CSV import |
CSV import |
||
| Line 70: | Line 70: | ||
[[Category:Organic reactions]] | [[Category:Organic reactions]] | ||
[[Category:Carbon-carbon bond forming reactions]] | [[Category:Carbon-carbon bond forming reactions]] | ||
<gallery> | |||
File:Aldolsimple.svg|Aldol reaction | |||
File:aldolrxnpic.jpg|Aldol reaction | |||
File:Aldol_addition_base-catalyzed.svg|Aldol reaction | |||
File:Aldol_addition_acid-catalyzed.svg|Aldol reaction | |||
File:Aldol_control_2_update.svg|Aldol reaction | |||
File:Aldol_control_3.svg|Aldol reaction | |||
File:Aldol_syn-anti.svg|Aldol reaction | |||
File:Enolate_metal_ion.svg|Aldol reaction | |||
File:Stereoselective_E-enolate_formation_of_esters_in_aldol_addtion_reactions.svg|Aldol reaction | |||
File:Stereoselective_Z-enolate_formation_of_esters_in_aldol_addtion_reactions.svg|Aldol reaction | |||
File:Enolate_alpha_center_eg.svg|Aldol reaction | |||
File:Enolate_alpha_center_model.svg|Aldol reaction | |||
</gallery> | |||
Latest revision as of 12:21, 18 February 2025
Aldol Reaction[edit]
The aldol reaction is a fundamental carbon-carbon bond-forming reaction in organic chemistry. It involves the reaction of an enolate ion with a carbonyl compound to form a _-hydroxy carbonyl compound, known as an aldol. This reaction is widely used in the synthesis of complex molecules and is a key step in many biosynthetic pathways.
Mechanism[edit]
The aldol reaction can proceed via two main mechanisms: base-catalyzed and acid-catalyzed.
Base-Catalyzed Aldol Reaction[edit]
In the base-catalyzed aldol reaction, a strong base such as sodium hydroxide or potassium hydroxide deprotonates the _-carbon of a carbonyl compound, generating an enolate ion. This enolate ion then attacks the carbonyl carbon of another molecule, forming a new carbon-carbon bond and resulting in a _-hydroxy carbonyl compound.

Acid-Catalyzed Aldol Reaction[edit]
In the acid-catalyzed aldol reaction, a proton is added to the carbonyl oxygen, increasing the electrophilicity of the carbonyl carbon. The enol form of the carbonyl compound then attacks the protonated carbonyl, leading to the formation of the aldol product.

Stereochemistry[edit]
The aldol reaction can produce different stereoisomers depending on the geometry of the enolate and the configuration of the newly formed stereocenters. The reaction can lead to syn or anti products, which are determined by the relative stereochemistry of the substituents.
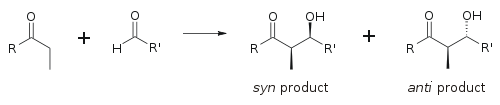
Enolate Formation[edit]
The formation of the enolate ion is a crucial step in the aldol reaction. The geometry of the enolate (E or Z) can influence the stereochemical outcome of the reaction.

Stereoselective Aldol Reactions[edit]
Stereoselective aldol reactions are designed to preferentially form one stereoisomer over another. This can be achieved by controlling the geometry of the enolate or by using chiral catalysts or auxiliaries.
E-Enolate Formation[edit]
The formation of E-enolates can be favored by using bulky bases or specific reaction conditions.

Z-Enolate Formation[edit]
Z-enolates can be formed under different conditions, often leading to different stereochemical outcomes.

Applications[edit]
The aldol reaction is used in the synthesis of many natural products and pharmaceuticals. It is a versatile reaction that allows for the construction of complex molecular architectures.
Related Pages[edit]
Gallery[edit]
-
Simple aldol reaction
-
Aldol reaction example
-
Aldol reaction control
-
Aldol reaction control
-
Enolate alpha center example
-
Aldol reaction
-
Aldol reaction
-
Aldol reaction
-
Aldol reaction
-
Aldol reaction
-
Aldol reaction
-
Aldol reaction
-
Aldol reaction
-
Aldol reaction
-
Aldol reaction
-
Aldol reaction
-
Aldol reaction

