Persistent carbene: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 20: | Line 20: | ||
{{Chem-stub}} | {{Chem-stub}} | ||
<gallery> | |||
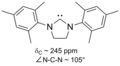
File:SIMes.png|Persistent_carbene | |||
File:Breslow-carbene.png|Persistent_carbene | |||
File:Wanzlick-1970.png|Persistent_carbene | |||
File:Bertrand1.png|Persistent_carbene | |||
File:Arduengo2.png|Persistent_carbene | |||
File:Tetramethyl_imidazol-2-ylidene.png|Persistent_carbene | |||
File:Bis(diisopropylamino)carbene.png|Persistent_carbene | |||
File:Alder2.png|Persistent_carbene | |||
File:Imidazol2ylidenes1.png|Persistent_carbene | |||
File:1,3-di-1-(2,4,6-mesityl)-4,5-dichloroimidazol-2-ylidene.png|Persistent_carbene | |||
File:Triazol-5-ylidenes.png|Persistent_carbene | |||
File:1,2,4-triazol-5-ylidenes.png|Persistent_carbene | |||
</gallery> | |||
Latest revision as of 11:59, 18 February 2025
Persistent carbene refers to a class of carbene compounds that exhibit unusual stability compared to the traditionally reactive nature of carbenes. Carbenes are a group of organic compounds characterized by the presence of a divalent carbon atom with two non-bonded electrons, making them highly reactive intermediates in organic chemistry. However, persistent carbenes, also known as stable carbenes, have a singlet ground state where the two electrons are paired but do not form a bond, leading to a configuration that is surprisingly resistant to dimerization and other forms of decomposition that are common among traditional carbenes.
Structure and Stability[edit]
The stability of persistent carbenes is attributed to several factors, including the presence of electron-donating groups adjacent to the carbene center, which helps to delocalize the lone pair of electrons on the carbene carbon, and the steric hindrance provided by bulky substituents that prevent the approach of potential reactants. These structural features are exemplified in well-known persistent carbenes such as imidazol-2-ylidenes, triazol-5-ylidenes, and diarylmethane-based carbenes.
Synthesis[edit]
Persistent carbenes are typically synthesized through the deprotonation of a precursor compound, such as an imidazolium salt for imidazol-2-ylidenes, using a strong base. The choice of precursor and base, as well as the reaction conditions, are crucial for the successful generation of the carbene without triggering its decomposition.
Applications[edit]
Due to their stability and unique electronic properties, persistent carbenes have found applications in various areas of chemistry. They are particularly valuable as ligands in transition metal catalysis, where they can stabilize metal centers and facilitate catalytic reactions. Persistent carbenes have also been explored as organocatalysts and in the development of new materials and polymers.
Comparison with Traditional Carbenes[edit]
Unlike traditional carbenes, which are highly reactive and short-lived, persistent carbenes can be isolated, stored, and handled under standard laboratory conditions. This remarkable stability opens up new avenues for research and application that were previously inaccessible with conventional carbene chemistry.
Future Directions[edit]
Research in the field of persistent carbenes continues to evolve, with efforts focused on exploring new structures, understanding their reactivity and mechanism of action, and expanding their application in organic synthesis, catalysis, and material science.
-
Persistent_carbene
-
Persistent_carbene
-
Persistent_carbene
-
Persistent_carbene
-
Persistent_carbene
-
Persistent_carbene
-
Persistent_carbene
-
Persistent_carbene
-
Persistent_carbene
-
Persistent_carbene
-
Persistent_carbene
-
Persistent_carbene