Wolff–Kishner reduction: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import |
||
| Line 33: | Line 33: | ||
{{Chemistry-stub}} | {{Chemistry-stub}} | ||
<gallery> | |||
File:Wolff-Kishner-reaction_scheme-new.png|Wolff–Kishner reduction | |||
File:Wolff-Kishner_mechanism-s.png|Wolff–Kishner reduction | |||
File:Kishner1.png|Wolff–Kishner reduction | |||
File:Wolff-new.png|Wolff–Kishner reduction | |||
File:Wolff-Kishner_mechanism-new.png|Wolff–Kishner reduction | |||
File:Huang-Minlon_modification.png|Wolff–Kishner reduction | |||
File:Barton1.png|Wolff–Kishner reduction | |||
File:Cram_modification1.png|Wolff–Kishner reduction | |||
File:Caglioti2.png|Wolff–Kishner reduction | |||
File:Caglioti-mechanism-new.png|Wolff–Kishner reduction | |||
File:Alternative_mechanism_for_caglioti_reaction-new.png|Wolff–Kishner reduction | |||
File:Caglioti_Reaction_Mechanism.png|Wolff–Kishner reduction | |||
</gallery> | |||
Latest revision as of 11:39, 18 February 2025
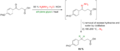
Wolff–Kishner Reduction is a chemical reaction that involves the complete reduction of a carbonyl group to a methylene group under strongly basic conditions. This reaction is significant in organic chemistry for the modification of ketones and aldehydes into alkanes, without affecting carbon-carbon double or triple bonds. The Wolff–Kishner reduction is particularly useful in synthetic organic chemistry for the preparation of complex molecules.
Overview[edit]
The reaction was independently discovered by Ludwig Wolff and Nikolai Kishner in the early 20th century. It is typically carried out by heating a carbonyl compound with hydrazine (N_2H_4) and a strong base, usually potassium or sodium hydroxide (NaOH or KOH), in a high-boiling solvent such as diethylene glycol. The mechanism involves the formation of a hydrazone, followed by a base-induced elimination of nitrogen, resulting in the formation of an alkane.
Mechanism[edit]
- Formation of Hydrazone: The carbonyl compound reacts with hydrazine to form a hydrazone.
- Base-induced Elimination: The strong base deprotonates the hydrazone, leading to the formation of a nitrogen anion, which then eliminates nitrogen gas, resulting in the formation of a carbanion.
- Protonation: The carbanion is protonated to form the final alkane product.
Variations[edit]
Several modifications of the Wolff–Kishner reduction have been developed to improve its applicability under milder conditions or in specific contexts. These include the Huang-Minlon modification, which is carried out in ethylene glycol at a lower temperature, and the Clemmensen reduction, which is an alternative method for reducing carbonyl compounds using zinc amalgam and hydrochloric acid.
Applications[edit]
The Wolff–Kishner reduction is widely used in the synthesis of complex organic molecules, including natural products and pharmaceuticals. Its ability to selectively reduce carbonyl groups without affecting other functional groups makes it a valuable tool in the chemist's arsenal.
Comparison with Other Reductions[edit]
The Wolff–Kishner reduction is often compared to the Clemmensen reduction, which also reduces carbonyl compounds to alkanes but operates under acidic conditions. The choice between these two methods depends on the sensitivity of the substrate to acidic or basic conditions.
See Also[edit]
- Hydrazine
- Sodium hydroxide
- Potassium hydroxide
- Clemmensen reduction
- Reduction reactions in chemistry
References[edit]
<references/>
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction
-
Wolff–Kishner reduction