Engineered CAR T cell delivery: Difference between revisions
CSV import Tags: mobile edit mobile web edit |
CSV import Tags: mobile edit mobile web edit |
||
| Line 26: | Line 26: | ||
[[Category:Cell therapy]] | [[Category:Cell therapy]] | ||
{{pharmacology-stub}} | {{pharmacology-stub}} | ||
<gallery> | |||
File:Peripherally_inserted_central_catheter_(PICC).png|Peripherally Inserted Central Catheter (PICC) | |||
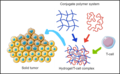
File:CAR-T_Gel_Delivery.png|CAR-T Gel Delivery | |||
File:Engineered_polymeric_microneedle_cellular_delivery.jpg|Engineered Polymeric Microneedle Cellular Delivery | |||
File:CAR_T-Cell_Therapy.jpg|CAR T-Cell Therapy | |||
</gallery> | |||
Latest revision as of 04:58, 18 February 2025
Engineered CAR T-cell delivery refers to the advanced methodologies and technologies used for administering Chimeric Antigen Receptor (CAR) T-cells into patients. This innovative approach is a cornerstone of immunotherapy, specifically in the treatment of various types of cancer. CAR T-cell therapy involves the genetic modification of a patient's T-cells to express a CAR that targets and destroys cancer cells. The process of engineered CAR T-cell delivery encompasses several critical steps, including T-cell collection, genetic modification, expansion, and infusion back into the patient.
Overview[edit]
CAR T-cell therapy represents a significant breakthrough in cancer treatment, offering hope for patients with certain types of blood cancers that have not responded well to other treatments. The success of CAR T-cell therapy largely depends on the efficiency and safety of the delivery systems used to introduce modified T-cells into the patient's body. Engineered CAR T-cell delivery aims to optimize these systems to improve patient outcomes.
T-cell Collection[edit]
The first step in CAR T-cell therapy is the collection of the patient's T-cells, typically through a process called leukapheresis. In this procedure, blood is drawn from the patient, and a machine separates out the T-cells, while the rest of the blood is returned to the patient's body.
Genetic Modification[edit]
Once collected, the T-cells are genetically modified in a laboratory to express the CAR, which is designed to recognize and bind to a specific antigen on the surface of cancer cells. This modification is usually achieved using viral vectors, although non-viral methods are also being explored.
T-cell Expansion[edit]
After modification, the CAR T-cells are cultured in the lab to increase their numbers. This expansion process is crucial to ensure that a sufficient number of CAR T-cells are available to effectively target and kill cancer cells once reintroduced into the patient.
Infusion[edit]
The final step involves infusing the expanded CAR T-cells back into the patient. This is typically done through an intravenous (IV) line. Once infused, the CAR T-cells begin to multiply within the patient's body and, more importantly, recognize and kill cancer cells expressing the target antigen.
Challenges and Innovations[edit]
Engineered CAR T-cell delivery faces several challenges, including managing the potential side effects, such as cytokine release syndrome (CRS) and neurotoxicity. Innovations in delivery techniques, such as the development of switchable CAR T-cells and the use of safety switches, aim to improve the safety and efficacy of CAR T-cell therapies.
Future Directions[edit]
Research in engineered CAR T-cell delivery continues to evolve, with efforts focused on expanding the applicability of CAR T-cell therapy to solid tumors, improving the safety profile of CAR T-cell therapies, and developing more efficient and less costly manufacturing and delivery processes.
-
Peripherally Inserted Central Catheter (PICC)
-
CAR-T Gel Delivery
-
Engineered Polymeric Microneedle Cellular Delivery
-
CAR T-Cell Therapy