Adenine phosphoribosyltransferase: Difference between revisions
CSV import |
CSV import |
||
| Line 41: | Line 41: | ||
[[Category:Metabolism]] | [[Category:Metabolism]] | ||
[[Category:Genetic disorders]] | [[Category:Genetic disorders]] | ||
<gallery> | |||
File:APRT-CpG.svg|Adenine phosphoribosyltransferase | |||
File:ARPTase_Reaction_Scheme.png|Reaction scheme of APRTase | |||
File:Flexible_loop_and_Hood_domains_of_human_APRTase.png|Flexible loop and Hood domains of human APRTase | |||
File:Human_APRTase,_adenine_binding_site.png|Adenine binding site of human APRTase | |||
</gallery> | |||
Latest revision as of 04:28, 18 February 2025
Adenine phosphoribosyltransferase[edit]
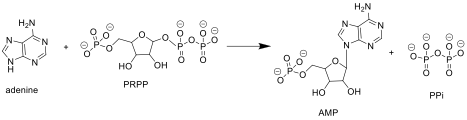
Adenine phosphoribosyltransferase (APRT) is an enzyme that plays a crucial role in the purine salvage pathway, which is essential for the recycling of purines to synthesize nucleotides. This enzyme catalyzes the conversion of adenine and phosphoribosyl pyrophosphate (PRPP) into adenosine monophosphate (AMP).
Function[edit]
APRT is responsible for the salvage of adenine, a purine base, by converting it into AMP. This reaction is important for maintaining the balance of purine nucleotides in the cell and for conserving energy by recycling purines rather than synthesizing them de novo.
Mechanism[edit]
The reaction catalyzed by APRT involves the transfer of a phosphoribosyl group from PRPP to adenine, forming AMP and pyrophosphate. This reaction is facilitated by the enzyme's active site, which binds both substrates and stabilizes the transition state.
Structure[edit]
APRT is a homodimeric enzyme, meaning it consists of two identical subunits. Each subunit contains a flexible loop and a hood domain that are important for substrate binding and catalysis.

Active Site[edit]
The active site of APRT is where adenine and PRPP bind. The enzyme undergoes conformational changes upon substrate binding, which facilitates the catalytic process. The binding site is highly specific for adenine, ensuring the correct substrate is utilized.

Genetic Implications[edit]
Mutations in the APRT gene can lead to a deficiency in the enzyme, resulting in the accumulation of adenine and its conversion to 2,8-dihydroxyadenine, which can cause kidney stones and renal failure. This condition is known as APRT deficiency.
Clinical Significance[edit]
APRT deficiency is a rare genetic disorder that can lead to the formation of kidney stones composed of 2,8-dihydroxyadenine. Early diagnosis and treatment are important to prevent kidney damage. Treatment typically involves a low-purine diet and medications that reduce adenine production.
Related pages[edit]
Gallery[edit]
-
CpG island of APRT gene.
-
Adenine phosphoribosyltransferase
-
Reaction scheme of APRTase
-
Flexible loop and Hood domains of human APRTase
-
Adenine binding site of human APRTase


